- Department of Neurosurgery, Centre Clinical, Chirurgie de Rachis, Soyaux, France
- Department of Neurosurgery, International Neurosciences Institut, Hannover, Germany
Correspondence Address:
Kevyan Mostofi
Department of Neurosurgery, International Neurosciences Institut, Hannover, Germany
DOI:10.4103/2152-7806.189437
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Mostofi K, Khouzani RK. Surface anatomy for implantation of external ventricular drainage: Some surgical remarks. Surg Neurol Int 29-Aug-2016;7:
How to cite this URL: Mostofi K, Khouzani RK. Surface anatomy for implantation of external ventricular drainage: Some surgical remarks. Surg Neurol Int 29-Aug-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/surface-anatomy-implantation-external-ventricular-drainage-surgical-remarks/
Abstract
Background:External ventricular drainage (EVD) is an emergency process intended to reduce intracranial hypertension resulting from the obstruction of cerebrospinal fluid (CSF) flow. This creates a temporary situation to extract CSF that cannot pass through normally. Knowing the surface anatomy for EVD implantation is important to prevent its inadvertent complications. The external landmarks have been designed in this anatomic study to review the classical landmarks and come up with new landmarks to improve this simple but lifesaving procedure.
Methods:From November 1998 to October 2012, we implanted 439 EVDs.
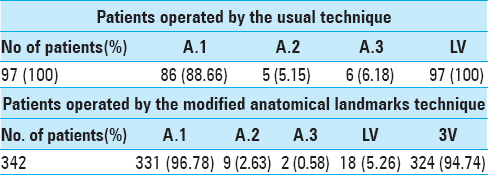
Results:In the first years, we employed usual landmarks to implant 97 EVDs. Since 2002, we used modified anatomical landmarks to implant 342 EVDs directly in the third ventricle.
Conclusion:Using effective landmarks for EVD implementation allows the catheter to be inserted in the third ventricle. In addition, it permits more precise accuracy to ensure a safer procedure with fewer complications.
Keywords: Acute hydrocephalus, cerebrospinal fluid, external ventricular derivation, lateral ventricle, third ventricle
INTRODUCTION
External ventricular drainage (EVD) is an emergency procedure aimed at reducing intracranial hypertension resulting from the obstruction of cerebrospinal fluid (CSF) flow. It is a relatively simple but lifesaving process. This process creates a temporary situation to extract CSF that cannot pass through normally.
The procedure consists of implementing a shunt in the right frontal horn of the lateral ventricle. It is usually done without any image guidance, which may be life-threatening.
The surgery is decided according to patient's clinical condition, his (her) clinical exam, and his (her) imaging [computed tomography (CT) scan or magnetic resonance imaging (MRI)].
Being considered a simple procedure, EVD implementation is made using surface anatomy. It is generally the first surgery carried out by young resident or neurosurgeons.
Although in a majority of instances lateral ventricle is dilated, the neurosurgeon is sometimes confronted by cases in which the frontal horn is normal even narrow. Therefore, knowledge of the cranium projections of brain structures is essential for the success of the surgery and reduces the surgical complications and misplaced catheter. With experience and by the logic and nature of diseases requiring the implementation of EVD, on one hand, and reflecting brain anatomy and the physiopathology of CSF circulation and absorption, on the other hand, we believe that the implementation of EVD in the third ventricle is the best way to have a situation as close to reality as possible.
This paper presents our experience, our knowledge acquired over the years, our remarks, and our conclusion about EVD implantation in the context of the literature.
MATERIALS AND METHODS
From November 1998 to October 2012, we implanted 439 EVDs. Pediatric cases were excluded from study. In the first years, we employed usual landmarks to implant 97 EVDs. Since 2002, we used modified anatomical landmarks to implant 342 directly in third ventricle.
According to usual landmarks, the patient is positioned in a dorsal decubitus, the head is slightly flexed with a 0 degree of rotation. The trepanation hole is made 2–3 cm lateral to the midline and 1–2 cm anterior to the coronal suture. The ventricular catheter is passed perpendicular to the brain. The surgeon enters the lateral ventricle at 5–6 cm from the calvarium.[
Since 2002, we employed the following technique and landmarks; for burr hole spacing, we choose a point 11.5 cm posterior to Nasion (measured by a flexible rule) at the midline. At this point, we choose a point 2.5 cm lateral to the midline and perpendicular to the latter. The burr hole is placed more or less at this point. The 3 cm curved incision is placed central to this point with its concave side facing anteriorly. We use a three-dimensional trajectory to first enter the lateral ventricle and then the third ventricle. For this purpose, we employ three references point in space. The first one is the inner canthus of the ipsilateral eye in the coronal plane. First, this point is targeted by the catheter. The second one is tragus in the sagittal plane. The trajectory is adjusted the second time to be parallel to tragus. And the third and last point in the space is the midpapillary point of the eye in axial plane. This point determines the exact placement of the burr hole that will be adjusted and referenced to the starting point (2.5 cm to midline). The burr hole is performed by twist drill. Dura is open by electric bistoury. The catheter is passed according to landmarks, point references, and abovementioned imaginary lines.
Once a slight resistance was felt, there will be immediately a popping as the ependymal layer is punctured. The catheter is pushed at 8.5–9.5 cm from calvarium to be placed gently in the third ventricle.
RESULTS
All the patients had a postoperative imaging (MRI or CT scan) at the latest 24 hours after surgery. If the patients did not improve or their neurological condition deteriorated, they had an imaging immediately after EVD implantation.
DISCUSSION
Over time, we modified our landmarks for EVD implantation. These modifications were carried out on the basis of the experience of EVD implementation but also, in particular, the experience of ventriculocysternostomy that need device introduction in the third ventricle. We noted that, with stringent compliance of landmarks and trajectories, we can enter the catheter in the third ventricle in the vast majority of cases without any difficulty. The interest of EVD implantation in the third ventricle is multiple; well-balanced extraction of CSF; non-unilateral evacuation, in particular, in the case of unilateral intracranial pressure due to tumor mass or other possible causes; and valve obstruction avoidance in case of lateral ventricular collapse. Obviously, there may be cases where EVD in the third ventricle is impossible. However, there have only been a handful of cases in our series.
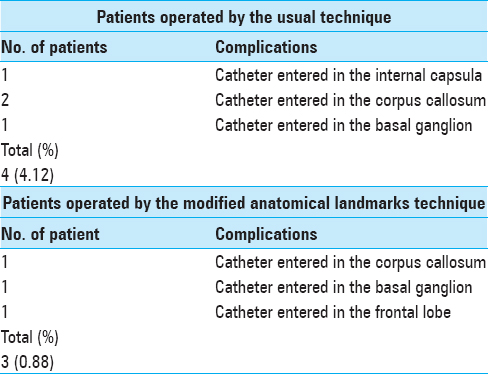
Our three-dimensional landmarks provide the most precise accuracy against usual two-dimensional landmarks, and even more because this surgery is generally carried out as an emergency and would not always be possible to use neuronavigation and stereotaxic frame. Despite its reputation as a relatively simple procedure, this surgery is not an innocuous act and several complications have been identified in the medical literature. In addition to the general complications of EVD implantation as hemorrhage and obstruction, the most common misplaced catheters are basal ganglion, thalamus, internal capsula, corpus callosum, and frontal lobe.[
In our series of 342 patients using three-dimensional landmarks, we had only 3 (0.88%) misplaced catheters [
Using usual technique, we were led to try a second even a third time to correctly place the catheter and see CSF flow in 11 patients (11.33%) against 11 patients (3.21%) who were operated by modified anatomical landmarks technique. In this regard, we do not believe that this results were due to lack of experience because the authors had already implanted more than 100 hundred EVDs before the first series.
Some remarks
This surgery is performed in a patient in a dorsal decubitus position. The patient's head is in a straight up, slightly flexed position. Care must be taken to not bend the head that would increase intracranial pressure. In general, the surgery is carried out on the right side.[
CONCLUSION
Using effective landmarks for EVD implementation allows the catheter to be inserted in the third ventricle. In addition, it permits more precise accuracy to ensure a safer procedure with less complications.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Bhargava D, Alalade A, Ellamushi H, Yeh J, Hunter R. Mitigating effects of external ventricular drain usage in the management of severe head injury. Acta Neurochir. 2013. 155: 2129-32
2. Chai FY, Farizal F, Jegan T. Coma due to malplaced external ventricular drain. Turk Neurosurg. 2013. 23: 561-3
3. Choo YS, Chung J, Joo JY, Kim YB, Hong CK. Borderline basal ganglia hemorrhage volume: Patient selection for good clinical outcome after stereotactic catheter drainage. J Neurosurg. 2016. p. 1-7
4. Cinibulak Z, Aschoff A, Apedjinou A, Kaminsky J, Trost HA, Krauss JK. Current practice of external ventricular drainage: A survey among neurosurgical departments in Germany. Acta Neurochir. 2016. 158: 847-53
5. Hsieh CT, Chen GJ, Ma HI, Chang CF, Cheng CM, Su YH. The misplacement of external ventricular drain by freehand method in emergent neurosurgery. Acta Neurol Belg. 2011. 111: 22-8
6. Kosty J, Pukenas B, Smith M, Storm PB, Zager E, Stiefel M. Iatrogenic vascular complications associated with external ventricular drain placement: A report of 8 cases and review of the literature. Neurosurgery. 2013. 72: 208-13
7. Meyer FB.editorsAtlas of Neurosurgery: Basic Approaches to Cranial and Vascular Procedures. Philadelphia: Churchill Livingstone; 1999. p.
8. Muirhead WR, Basu S. Trajectories for frontal external ventricular drain placement: Virtual cannulation of adults with acute hydrocephalus. Br J Neurosurg. 2012. 26: 710-6
9. Muralidharan R. External ventricular drains: Management and complications. Surg Neurol Int. 2015. 6: S271-4
10. Patil V, Lacson R, Vosburgh KG, Wong JM, Prevedello L, Andriole K. Factors associated with external ventricular drain placement accuracy: Data from an electronic health record repository. Acta Neurochir. 2013. 155: 1773-9
11. Phillips SB, Delly F, Nelson C, Krishnamurthy S. Bedside external ventricular drain placement: Can multiple passes be predicted on the computed tomography scan before the procedure?. World Neurosurg. 2014. 82: 739-44
12. Prabhakar H, Ali Z, Rath GP, Bithal PK. Tension pneumocephalus following external ventricular drain insertion. J Anesth. 2008. 22: 326-7
13. Roitberg BZ, Khan N, Alp MS, Hersonskey T, Charbel FT, Ausman JI. Bedside external ventricular drain placement for the treatment of acute hydrocephalus. Br J Neurosurg. 2001. 15: 324-7
14. Rosenbaum BP, Wheeler AM, Krishnaney AA. External ventricular drain placement causing upgaze palsy: Case report. Clin Neurol Neurosurg. 2013. 115: 1514-6
15. Sarrafzadeh A, Smoll N, Schaller K. Guided (VENTRI-GUIDE) versus freehand ventriculostomy: Study protocol for a randomized controlled trial. Trials. 2014. 15: 478-
16. Toma AK1, Camp S, Watkins LD, Grieve J, Kitchen ND. External ventricular drain insertion accuracy: Is there a need for change in practice?. Neurosurgery. 2009. 65: 1197-200
17. Walker CT, Stone JJ, Jacobson M, Phillips V, Silberstein HJ. Indications for pediatric external ventricular drain placement and risk factors for conversion to a ventriculoperitoneal shunt. Pediatr Neurosurg. 2012. 48: 342-7
18. Wong FW. Cerebrospinal fluid collection: A comparison of different collection sites on the external ventricular drain. Dynamics. 2011. 22: 19-24
19. Weisenberg SH, TerMaath SC, Seaver CE, Killeffer JA. Ventricular catheter development: Past, present, and future. J Neurosurg. 2016. 4: 1-9
20. Zhao B, Tan X, Yang H, Zheng K, Li Z, Xiong Y. Stent-assisted coiling versus coiling alone of poor-grade ruptured intracranial aneurysms: A multicenter study. J Neurointerv Surg. 2016. p.