- Department of Surgery and Anatomy, Division of Neurosurgery, Otorhinolaryngology and Head and Neck Surgery, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, São Paulo, Brazil.
- Department of Medical Image, Hematology and Clinical Oncology Otorhinolaryngology and Head and Neck Surgery, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, São Paulo, Brazil.
- Department of Ophthalmology, Otorhinolaryngology and Head and Neck Surgery, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, São Paulo, Brazil.
Correspondence Address:
Benedicto Oscar Colli, Department of Surgery and Anatomy, Division of Neurosurgery, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, São Paulo, Brazil.
DOI:10.25259/SNI_651_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Benedicto Oscar Colli1, Carlos Gilberto Carlotti Junior1, Ricardo Santos de Oliveira1, Guilherme Gozzoli Podolski Gondim, Daniel Giansanti Abud2, Eduardo Tanaka Massuda3, Francisco Veríssimo de Melo Filho3, Koji Tanaka1. Surgical management of embolized jugular foramen paragangliomas without facial nerve transposition: Experience of a public tertiary hospital in Brazil. 30-Sep-2021;12:482
How to cite this URL: Benedicto Oscar Colli1, Carlos Gilberto Carlotti Junior1, Ricardo Santos de Oliveira1, Guilherme Gozzoli Podolski Gondim, Daniel Giansanti Abud2, Eduardo Tanaka Massuda3, Francisco Veríssimo de Melo Filho3, Koji Tanaka1. Surgical management of embolized jugular foramen paragangliomas without facial nerve transposition: Experience of a public tertiary hospital in Brazil. 30-Sep-2021;12:482. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=11146
Abstract
Background: Jugular foramen paragangliomas (JFP) treatment represents a challenge for surgeons due to its close relationship with facial nerve (FN), lower cranial nerves (LCN), and internal carotid artery. Due to its hypervascularization, preoperative tumor embolization has been indicated.
Methods: Retrospective analysis of the clinical evolution of 26 patients with JFP class C/D previously embolized treated through infratemporal/cervical access without FN transposition.
Results: Total and subtotal resections were 50% each, regrowth/recurrence were 25%, and 23%, respectively, and mortality was 3.9%. Postoperatively, 68.4% of patients had FN House and Brackmann (HB) Grades I/II. New FN deficits were 15.4% post embolization and 30.7% postoperatively. Previous FN deficits worsened in 46.1%. Tumor involved the FN in 30.8% and in 62.5% of them these nerves were resected and grafted (60% of them had HB III). Lateral fall, ear murmur, and vertigo improved in all patients. Tinnitus improved in 77.8% and one patient developed tinnitus after surgery. Hearing loss did not improve, eight partial hearing loss remained unchanged and four worsened. New postoperative LCN deficits were 64.3%. Postoperative KPS between 80 and 100 dropped 8.3%. Two patients with secretory paragangliomas with arterial hypertension difficult to control had better postoperative blood pressure control.
Conclusion: Although still with significant morbidity due to FN and LCN injuries, the treatment of patients with JFP Fisch C/D has good long-term results. Surgical techniques without FN transposition have less intraoperative nerve damage, lower rates of total resection, and higher recurrence. Preoperative embolization of JFP reduces the intraoperative blood loss but can cause FN deficit.
Keywords: Clinical outcome, Jugular foramen paraganglioma, Preoperative embolization, Surgical treatment without facial nerve transposition
INTRODUCTION
Jugular foramen paragangliomas (JFP) constitute 22% of head and neck paragangliomas, 8.6% of temporal bone tumors and 80% of jugular foramen (JF) tumors.[
Technical details such as the anterior transposition of the facial nerve (FN) have been recommended,[
We retrospectively analyzed the clinical evolution of a series of 26 patients with Fisch class C/D[
MATERIALS AND METHODS
Patient population
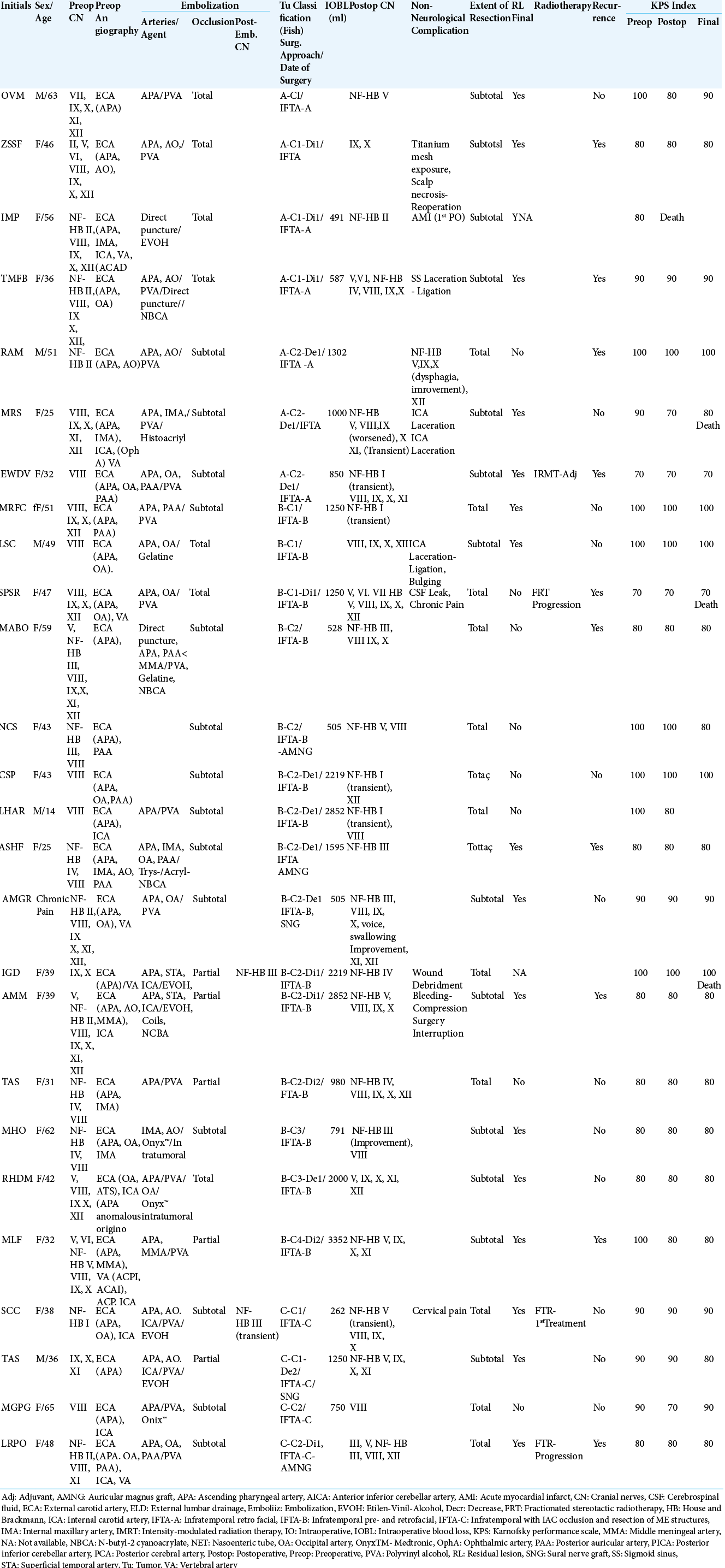
This study is a retrospective analysis of 26 patients with diagnosis of JFP surgically treated at the Division of Neurosurgery, Hospital das Clínicas, Ribeirão Preto Medical School, University of São Paulo by a multidisciplinary team, from February 2006 to January 2020. The study was approved by the Research Ethics Committee of our institution (No. 736,988). The diagnosis was made using non enhanced and enhanced brain and temporal bone computed tomography (CT) and magnetic resonance imaging (MRI) and confirmed by histopathological examination. The epidemiological characteristics and clinical manifestations are shown in [
All patients were submitted to urine metanephrine dosages and more recently also to dosage of catecholamines and vanillylmandelic acid. Two patients who had arterial hypertension difficult to control had increased metanephrine dosage requiring preoperative beta-blockers administration.
Surgical treatment
The patients underwent microsurgery, using four variations of the intrabulbar infratemporal approach (IFTA)-A[
Surgical technique
The technique used was reported early,[
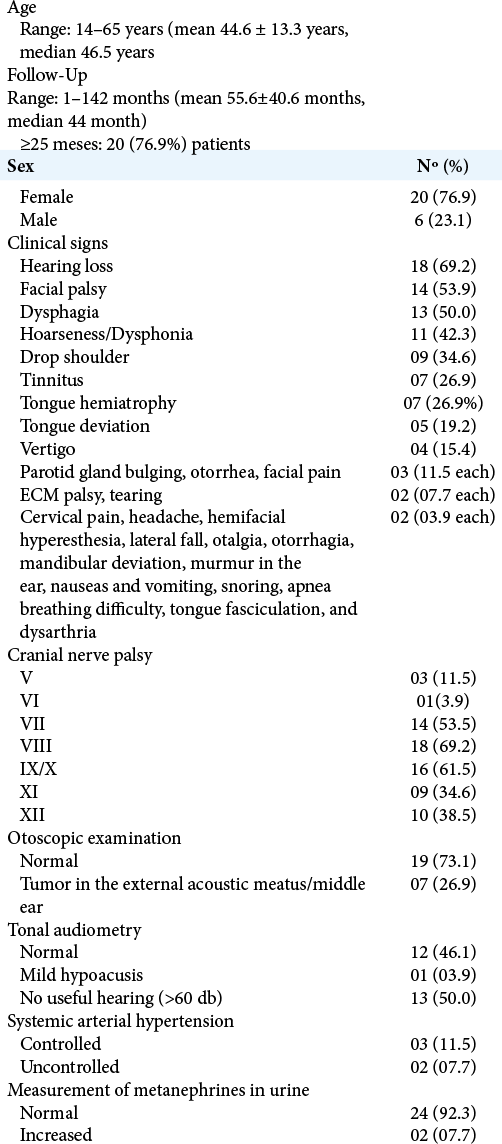
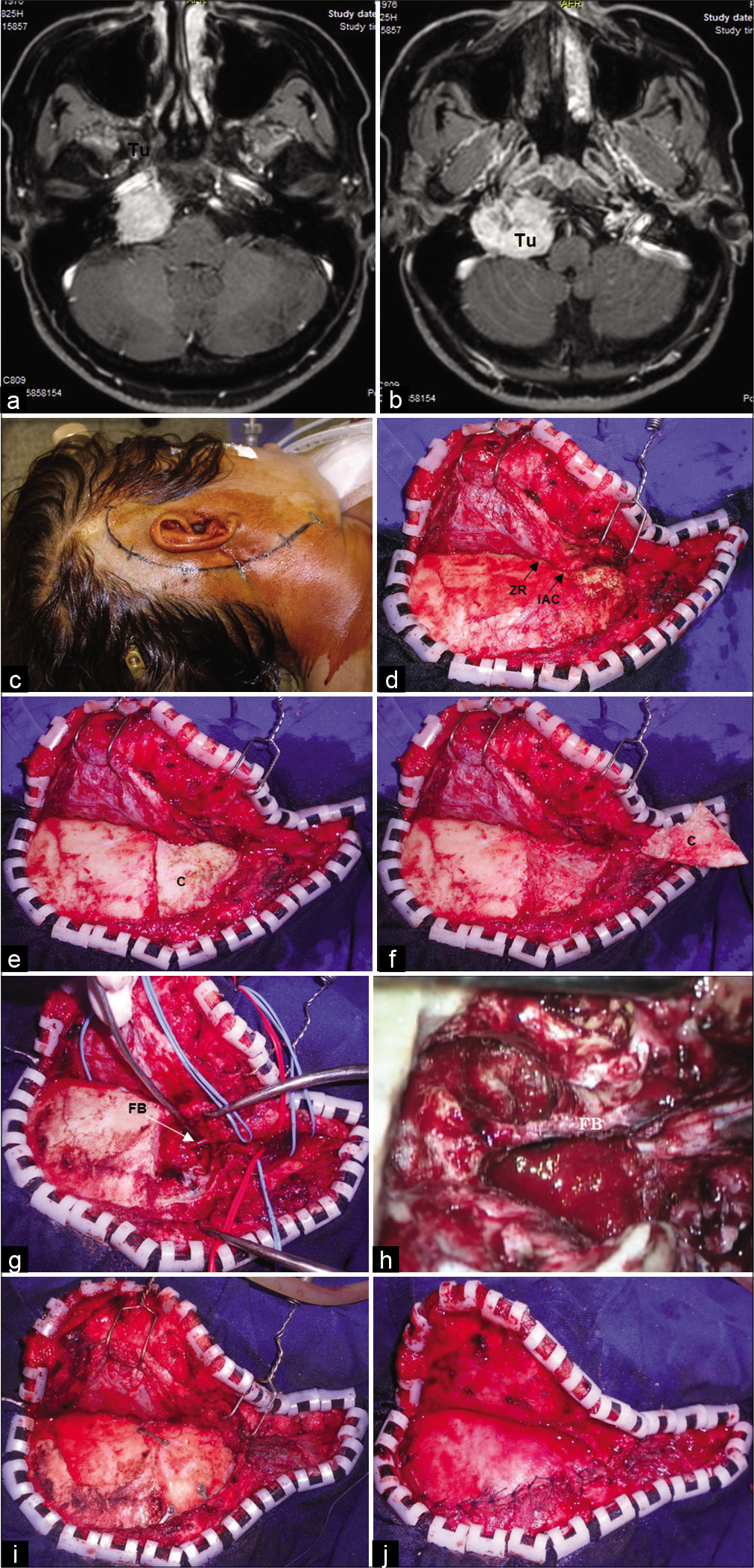
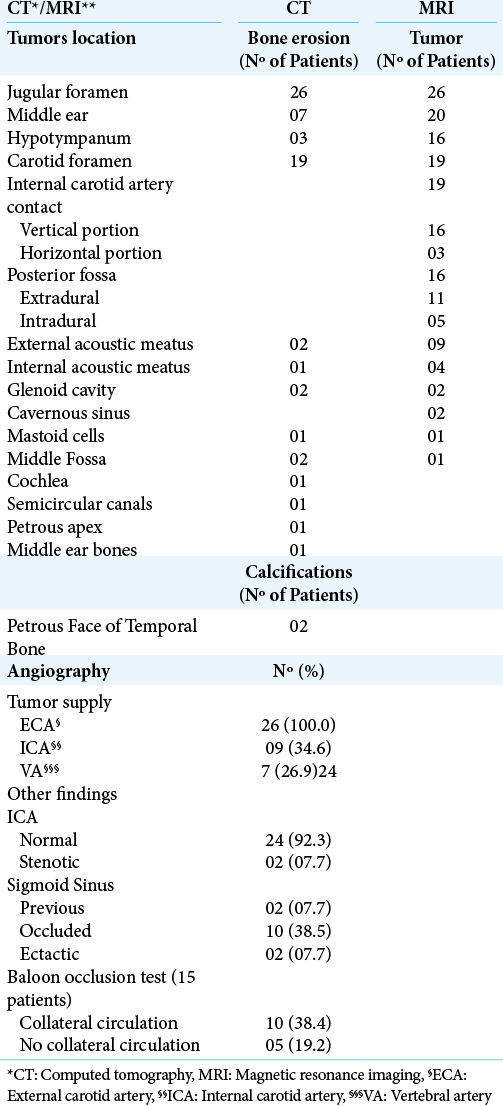
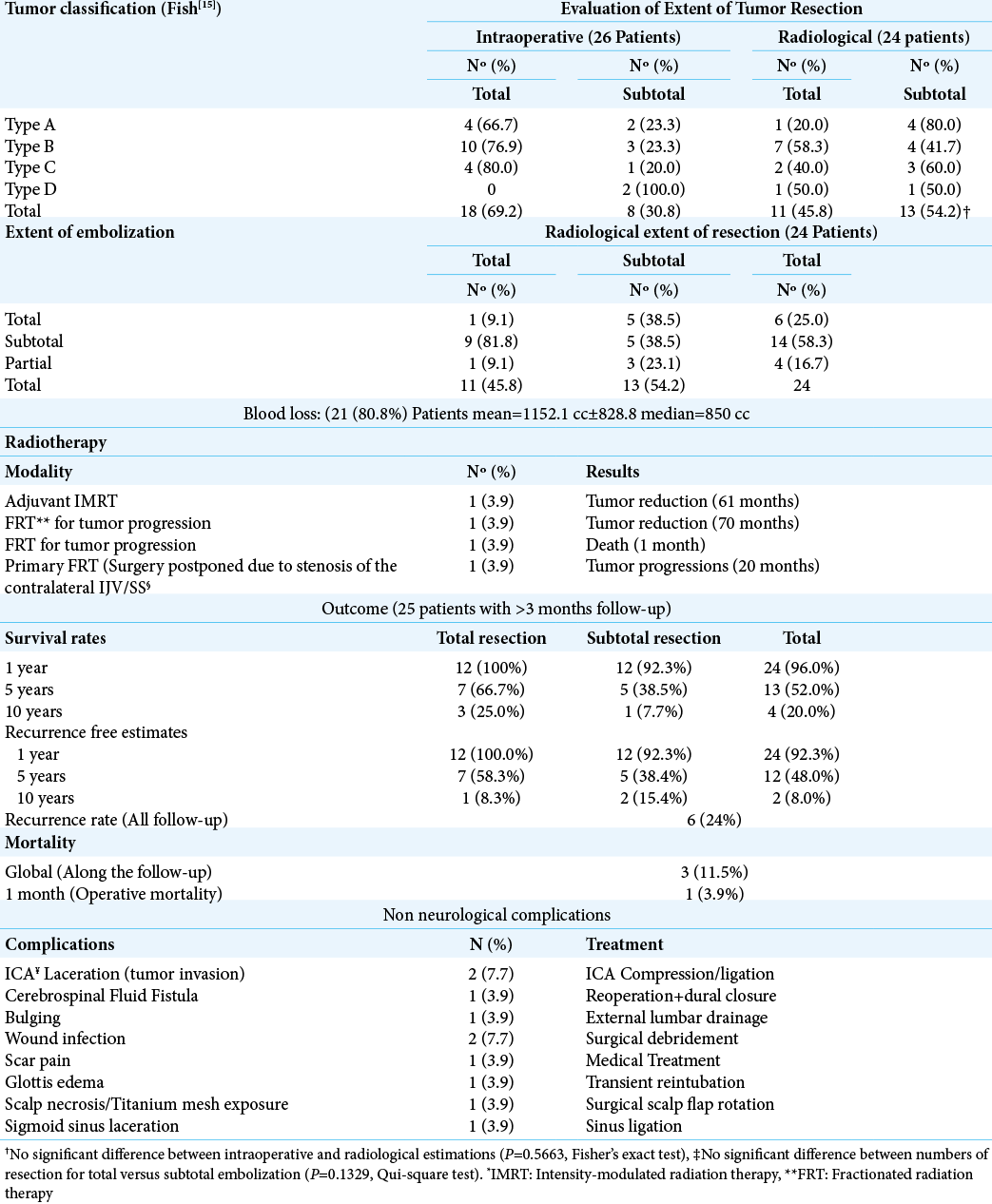
Figure 1:
Steps for surgical approach of an infratemporal (IFTA-B) and cervical approach of a paraganglioma of the jugular foramen (JF) C3. (a and b) Preoperative axial T1-weighted contrast-enhanced MR images showing a large mass (Tu) located in the JF with extension to the cervical region and posterior fossa, pushing the dura-mater. (c) Skin incision. (d) Exposure of the temporal bone and mastoid process after anterior displacement of the skin and of the fascio/muscular (temporal and sternocleidomastoid muscles) flaps. (e) Cortical bone of the mastoid (c) separated and keep in place. (f) Cortical bone (c) of the mastoid displaced. (g) Mastoidectomy with a fallopian bridge (FB) and cervical exposure. (h) Magnification of the fallopian bridge (FP) after bone removal. (i) Cortical of the mastoid replaced and fixed with mini-plates and screws. (j) Suture of the fascio-muscular plane.
Facial and LCN monitoring was used routinely since 2015 (last 12 cases) and auditory evoked potential is used for patients that have useful hearing. No preoperative traqueostomy or gastrostomy was preoperative used and no prophylactic lumbar external drainage was routinely used even in cases of intradural extension of the tumor.
Extension of resection
The extension of resection was estimated macroscopically during surgery and by a neuroradiologist (pre- and postoperative CT and/or MRI comparisons). Recurrence/ progression was assessed radiologically in the first 3 and 6 postoperative months and then annually/biannually.
Clinical outcome
The evolution of cranial nerve (CN) deficits and the Karnofsky Performance Scale (KPS) index were evaluated. FN lesions were classified according to the HB scale. KPS indices were obtained before, 3 months after surgery and at the last follow-up. Global survival (GS) and recurrence-free survival (RFS) curves were also assessed.
Statistical analysis
Chi-square and Fisher tests were used to compare proportions and the log-rank test (Mantel-Cox) was used to compare curves and survival estimates. Error probabilities not greater than 5% were considered significant for two-tailed probability tests. The tests were performed using the GraphPad PRISM program (version 9.0.0; GraphPad Software, Inc., San Diego, CA, USA).
RESULTS
[
Based on the CT/MRI images, the JFP was classified according to Fisch[
Surgical treatment
The results of the surgical treatment are summarized in [
Table 3:
Treatment of 26 patients with glomus jugulare paragangliomas according Borba et al., 2010[
The overall recurrence rate was 25%, and the progression rate was 23.1%. Operative and overall mortality were 3.9% and 11.5%, respectively.
Non-neurological complications occurred in 9 (34.6%) patients [
CN outcome
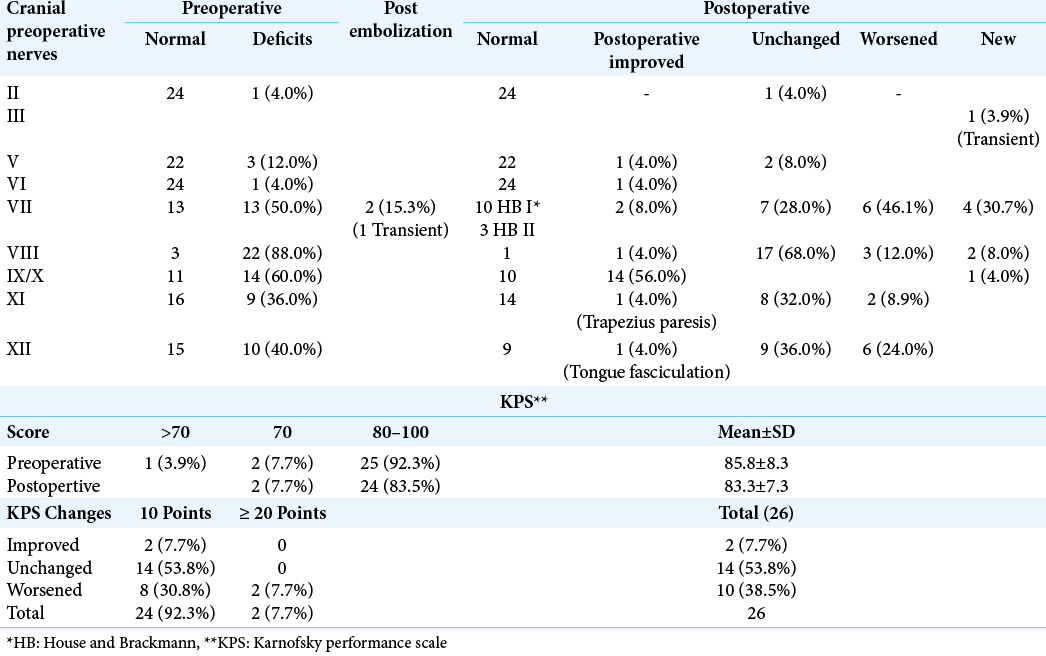
The CN deficits in 25 patients with follow-up ≥3 months are shown in [
Thirteen (50%) patients had preoperative FN deficits. Good FN function was observed in 68.4% (10 HB 1 and 3 HB II), 5 had HB III, 2 HB IV, and 7 HB V. New postoperative FN deficits occurred in 30.7%, worsened of the previous deficits occurred in 46.1% and improvement occurred in 15.4%. Tumoral involvement of the FN occurred 28.6%; 62.5% of them was grafted (two patients with sural and three with auricular nerves) and 60% reach HB III and 40% HB V. Two patients (15.4%), one sub totally and the other partially embolized, had FN lesions after embolization, one of which was transient.
New postoperative VIII CN deficits occurred in 8%. Lateral fall, ear murmur and vertigo improved in all patients. Tinnitus improved in 77.8% and postoperatively it occurred in one patient. Hearing loss improved in 4.6%, remained unchanged in 77.3% and worsened in 13.6%.
Nine patients (64.3%) had new postoperative LCN deficits, one from IX/X, 2 from XI, and 6 from XII CN [
The evolution of the KPS indices is shown in [
Both patients with secretory paragangliomas with arterial hypertension have better postoperative blood pressure control.
Radiotherapy
Three (11.5%) patients underwent fractionated stereotactic radiotherapy, two as rescue treatment and one as the first treatment (surgery was postponed due to stenosis of the contralateral sigmoid sinus); it was performed after post radiation tumor progression and verification of collateral circulation through the paravertebral plexus. A fourth patient underwent adjuvant intensity-modulated radiation therapy [
DISCUSSION
JFP is an uncommon tumor, usually benign, that can cause important neurological deficits due to its location.[
More effective and safer resections have been achieved with the use of microsurgical techniques, intraoperative electrophysiological monitoring and better understanding of the temporal bone anatomy. The surgical technique used in this series was IFTA-D,[
Fisch C/D class JFP resection is performed classically by infratemporal lateral approaches involving mastoidectomy and cervical exposure and eventually the posterior fossa. The approach to tumors that invade the tympanic cavity (ME and hypotympanum) through the lateral route has the FN and the ICA as obstacles. The transposition of the FN from its bone canal to the parotid region[
Preoperative angiography is essential to check tumor involvement and supply by ICA involvement and to check for the presence of collateral circulation. As these are hypervascularized lesions, preoperative intravascular[
LCN irrigation comes from the neuromeningeal branch of the ascending posterior artery (APA) through its jugular branches in the stylomastoid foramen (glossopharyngeal, vagus, and accessory nerves branches) and hypoglossal canal (hypoglossal nerve branch).[
The extension of resection and recurrence of JFP depend on the aggressiveness of the surgical technique and of the follow-up duration. In the first decade of this century, total resection varied from 51% to 92% and recurrence varied from 0% to 27.8%.[
We did not observe any difference between GS and RFS in relation to total and subtotal resections. Macroscopic intraoperative assessment of the extension of resection of JFP is not reliable because of bone invasion. The independent radiological evaluation showed a 20% decrease in the total resection rate.
Postoperative evolution of CNs
Signs of CN compression and irritation in patients with JFP Fisch C/D usually improve after surgery, but deficits do not always improve and may worsen. We observed improvement in hemifacial pain and motor deficit of the V CN and paresis of the VI CN after cavernous sinus decompression; nevertheless, hypoesthesia/hemifacial dysesthesia remained unchanged.
Tinnitus and hearing loss in patients with Fisch C/D class JFP, improved postoperatively in 37.3% and 6.6-29.4%, respectively, and worsening occurs in 2% and 0–50%, respectively.[
New postoperative FN deficits occur in 5.8-55.4% for JFP.[
LCN injuries occur in 0–67% of patients with JFP treated surgically.[
The operative mortality of patients with JFP operated is relatively low (0–2.6%).[
Radiotherapy
Recent systematic reviews of the literature show good tumor control in the primary treatment of jugulo-tympanic paragangliomas with stereotactic radiosurgery (STR) and Gamma-knife and good effectiveness and fewer complications when compared to adjunctive surgery or isolated microsurgical treatment.[
This study exhibits and presents the limitations that are inherent of a retrospective study, which that must be considered in its interpretation of the results. In addition, despite the evidence in the literature about the better results of postoperative FN function without transposition, we have no own series of patient with FN transposition to compare.
CONCLUSION
The treatment of patients with JFP Fisch C/D, although still with morbidity, has good long-term results. Surgical techniques without the transposition of the FN cause fewer intraoperative lesions; however, it also causes lower rates of total resection and higher recurrence. Preoperative embolization of JFP reduces the intraoperative blood loss but can cause FN deficit. Our results indicate that JFP can be treated with good results in general hospitals using parsimonious preoperative embolization and the posterior and anterior approach to the FN (fallopian bridge) approach, if a multidisciplinary team is available. Rescue SRS in cases of recurrence should be considered in each case, depending on the characteristics of patients and tumors.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Al-Mefty O, Fox JL, Rifai A, Smith RR. A combined infratemporal and posterior fossa approach for the removal of giant glomus tumors and chondrosarcomas. Surg Neurol. 1987. 28: 423-31
2. Al-Mefty O, Teixeira A. Complex tumors of the glomus jugulare: Criteria, treatment, and outcome. J Neurosurg. 2002. 97: 1356-66
3. Bacciu A, Prasad SC, Sist N, Rossi G, Piazza P, Sanna M. Management of the cervico-petrous internal carotid artery in class C tympanojugular paragangliomas. Head Neck. 2016. 38: 899-905
4. Borba LA, Araújo JC, de Oliveira JG, Filho MG, Moro MS, Tirapelli LF. Surgical management of glomus jugulare tumors: A proposal for approach selection based on tumor relationships with the facial nerve. J Neurosurg. 2010. 112: 88-98
5. Brackman D, Kinney S, Fu K. Glomus tumor: Diagnosis and management. Head Neck Surg. 1987. 9: 306-11
6. Capps FC. Glomus jugulare tumours of the middle ear. J Laryngol Otol. 1952. 66: 302-14
7. Catapano JS, Al-Mefty RO, Ding D, Whiting AC, Pines AR, Richter KR. Onyx embolization of skull base paragangliomas: A single-center experience. Acta Neurochir (Wien). 2020. 162: 821-9
8. Contrera J, Yong Reddy CA, Liu SW, Lorenz RR. Recurrence and progression of head and neck paragangliomas after treatment. Otolaryngol Head Neck Surg. 2020. 162: 504-11
9. Djindjian R, Merlandd JJ.editors. Normal super-selective arteriography of the external carotid artery. Super-Selective Arteriography of the External Carotid Artery. New York, Berlin: Springer-Verlag; 1978. p. 1-36
10. Elhammady MS, Peterson EC, Johnson JN, Aziz-Sultan MA. Preoperative onyx embolization of vascular head and neck tumors by direct puncture. World Neurosurg. 2012. 77: 725-30
11. Erickson D, Kudva YC, Ebersold MJ, Thompson GB, Grant CS, van Heerden JA. Benign paragangliomas: Clinical presentation and treatment outcomes in 236 patients. J Clin Endocrinol Metab. 2001. 86: 5210-6
12. Fayad JN, Keles B, Brackmann DE. Jugular foramen tumors: Clinical characteristics and treatment outcomes. Otol Neurotol. 2010. 31: 299-305
13. Fisch U. Infratemporal fossa approach to tumours of the temporal bone and base of the skull. J Laryngol Otol. 1978. 92: 949-67
14. Fisch U. Current surgical treatment of intratemporal facial palsy. Clin Plast Surg. 1979. 6: 377-88
15. Fisch U.editors. Infratemporal fossa approach for lesions in the temporal bone and base of the skull. Neuro-Otology and Skull Base Surgery. 1984. p. 254-66
16. Gardner G, Cocke EW, Robertson JT, Trumbull ML, Palmer RE. Glomus jugulare tumours-combined treatment: Part I. J Laryngol Otol. 1981. 95: 437-54
17. Gartrell BC, Hansen MR, Gantz BJ, Gluth MB, Mowry SE, Aagaard-Kienitz BL. Facial and lower cranial neuropathies after preoperative embolization of jugular foramen lesions with ethylene vinyl alcohol. Otol Neurotol. 2012. 33: 1270-5
18. Gaynor BG, Elhammady ME, Angeli SI, Aziz-Sultan MA. Incidence of cranial nerve palsy after preoperative embolization of glomus jugulare tumors using onyx. J Neurosurg. 2014. 120: 377-81
19. Gottfried ON, Liu JK, Couldwell WT. Comparison of radiosurgery and conventional surgery for the treatment of glomus jugulare tumors. Neurosurg Focus. 2004. 17: E4
20. Ivan ME, Sughrue ME, Clark AJ, Kane AJ, Aranda D, Barani IJ. A meta-analysis of tumor control rates and treatment-related morbidity for patients with glomus jugulare tumors. J Neurosurg. 2011. 114: 1299-305
21. Jansen TT, Kaanders JH, Beute GN, Timmers HJ, Marres HA, Kunst HP. Surgery, radiotherapy or a combined modality for jugulotympanic paraganglioma of Fisch Class C and D. Clin Otolaryngol. 2018. 43: 1566-72
22. Jansen TT, Timmer JL, Marres HA, Kaanders JH, Kunst HP. Results of a systematic literature review of treatment modalities for jugulotympanic paraganglioma, stratified per Fisch class. Clin Otolaryngol. 2018. 43: 652-61
23. Li D, Zeng XJ, Hao SY, Wang L, Tang J, Xiao XR. Less-aggressive surgical management and long-term outcomes of jugular foramen paragangliomas: A neurosurgical perspective. J Neurosurg. 2016. 125: 1143-54
24. Makiese O, Chibbaro S, Marsella M, Ba Huy PT, George B. Jugular foramen paragangliomas: Management, outcome and avoidance of complications in a series of 75 cases. Neurosurg Rev. 2012. 35: 185-94
25. Marangos N, Schumacher M. Facial palsy after glomus jugulare tumour embolization. J Laryngol Otol. 1999. 113: 268-70
26. Nicoli TK, Sinkkonen ST, Anttila T, Mäkitie A, Jero J. Jugulotympanic paragangliomas in Southern Finland: A 40-year experience suggests individualized surgical management. Eur Arch Otorhinolaryngol. 2017. 274: 389-97
27. Nonaka Y, Fukushima T, Watanabe K, Friedman AH, McElveen JT, Cunningham CD. Less invasive transjugular approach with Fallopian bridge technique for facial nerve protection and hearing preservation in surgery of glomus jugulare tumors. Neurosurg Rev. 2013. 36: 579-86
28. Odat H, Shin SH, Odat MA, Alzoubi F. Facial nerve management in jugular paraganglioma surgery: A literature review. J Laryngol Otol. 2016. 130: 219-24
29. Ozanne A, Pereira V, Krings T, Toulgoat F, Lasjaunias P. Arterial vascularization of the cranial nerves. Neuroimaging Clin N Am. 2008. 18: 431-9
30. Pensak M, Jackler RK. Removal of jugular foramen tumors: The fallopian bridge technique. Otolaryngol Head Neck Surg. 1997. 117: 586-91
31. Piras G, Mariani-Constantine R, Sanna M. Are ouctomes of radiosurgery for tympanojugular paraganglioma overestimated?. Otol Neurotol. 2019. 40: 688-9
32. Prasad SC, Mimoune HA, Khardaly M, Piazza P, Russo A, Sanna M. Strategies and long-term outcomes in the surgical management of tympanojugular paragangliomas. Head Neck. 2016. 38: 871-85
33. Ramina R, Maniglia JJ, Fernandes YB, Paschoal JR, Pfeilsticker LN, Neto MC. Tumors of the jugular foramen: Diagnosis and management. Neurosurgery. 2005. 57: 59-68
34. Rustemi O, Raneri F, Volpin L, Iannucci G. Complete embolization of jugular paragangliomas by direct puncture. Technical note. Br J Neurosurg. 2019. 33: 328-31
35. Sahyouni R, Mahboubi H, Moshtaghi O, Goshtasbi K, Sahyouni S, Lin HW. Radiosurgery of glomus tumors of temporal bone: A meta-analysis. Otol Neurotol. 2018. 39: 488-93
36. Shapiro S, Kellermeyer B, Ramadan J, Jones G, Wiseman B, Cassis A. Outcomes of primary radiosurgery treatment of glomus jugulare tumors. Otol Neurotol. 2018. 39: 1079-87
37. Sinvalingam S, Konishi M, Shin SH, Ahmed RA, Piazza P, Sanna M. Surgical management of tympanojugular paragangliomas with intradural extension, with a proposed revision of the Fisch classification. Audiol Neurootol. 2012. 17: 243-55
38. Suárez C, Rodrigo JP, Bödeker CC, Llorente JL, Silver CE, Jansen JC. Jugular and vagal paragangliomas: Systematic study of management with surgery and radiotherapy. Head Neck. 2013. 35: 1195-204
39. White BD, Link MJ, Cloft HJ. Endovascular embolization of paragangliomas: A safe adjuvant to treatment. J Vasc Interv Neurol. 2008. 1: 37-41