- Department of Neurosurgery, Kentucky College of Osteopathic Medicine, University of Pikeville, Pikeville, Kentucky, United States.
- Department of Neurological Surgery, University of California, Irvine, California, United States.
- Department of Neurosurgery, College of Osteopathic Medicine, Kansas City University, Kansas City, Missouri, United States.
- Department of Neurosurgery, Rowan University School of Osteopathic Medicine, Stratford, New Jersey, United States.
- Department of Neurosurgery, University of Colorado School of Medicine, Aurora, Colorado, United States.
- Department of Neurosurgery, Neurology, Radiology and Neuroscience, University of Kentucky, Lexington, Kentucky, United States.
Correspondence Address:
Taylor Reardon, Kentucky College of Osteopathic Medicine, University of Pikeville, Pikeville, Kentucky, United States.
DOI:10.25259/SNI_667_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Taylor Reardon1, Morgan Turnow1, Sidney Elston1, Nolan J. Brown2, Gretchen M. Koller3, Shelly Sharma4, Michael W. Kortz5, Ahmed Mohyeldin2, Justin F. Fraser6. Surgical management of petrous apex cholesteatomas in the pediatric population: A systematic review. 28-Oct-2022;13:494
How to cite this URL: Taylor Reardon1, Morgan Turnow1, Sidney Elston1, Nolan J. Brown2, Gretchen M. Koller3, Shelly Sharma4, Michael W. Kortz5, Ahmed Mohyeldin2, Justin F. Fraser6. Surgical management of petrous apex cholesteatomas in the pediatric population: A systematic review. 28-Oct-2022;13:494. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=11961
Abstract
Background: Cholesteatomas are growths of squamous epithelium that can form inside the middle ear and mastoid cavity and damage nearby structures causing hearing loss when located at the petrous apex. The primary goal of petrous apex cholesteatoma resection is gross total removal with tympanoplasty and canal-wall up or canal-wall down tympanomastoidectomy. At present, there is no definitive surgical approach supported by greater than level 4 evidence in the literature to date.
Methods: A systematic review was conducted utilizing PubMed, Embase, and Scopus databases. Articles were screened and selected to be reviewed in full text. The articles that met inclusion criteria were reviewed for relevant data. Data analysis, means, and standard deviations were calculated using Microsoft Excel.
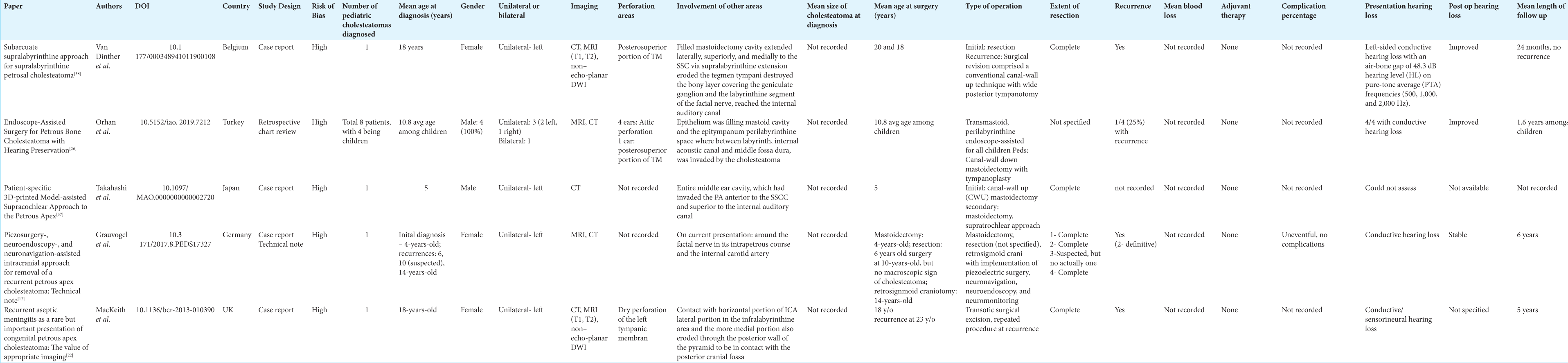
Results: After screening, five articles were included in the systematic review. There were a total of eight pediatric patients with nine total cholesteatomas removed. Conductive hearing loss was the most common (77%) presenting symptom. Perforations were noted in seven ears (86%). Recurrence was noted in 50% of patients with an average recurrence rate of 3.5 years (SD = 1.73). Average length of follow-up was 32.6 months (SD = 21.7). Canal-wall up was the most utilized technique (60%) and there were zero noted surgical complications. Five of the seven (71%) patients that experienced hearing loss from perforation noted improved hearing.
Conclusion: Due to its rarity, diagnostic evaluation and treatment can vary. Further, multi-institutional investigation is necessary to develop population-level management protocols for pediatric patients affected by petrous apex cholesteatomas.
Keywords: Cholesteatoma, Petrous apex, Skull base, Pediatric neurosurgery
INTRODUCTION
Cholesteatomas are abnormal growths of keratinizing squamous epithelium arising within the middle ear and mastoid cavity.[
MATERIALS AND METHODS
A systematic search for eligible literature describing pediatric cholesteatoma at the petrous apex was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analysis 2020 guidelines.[
RESULTS
A review of the literature yielded five articles that met inclusion criteria,[
DISCUSSION
We present a systematic and narrative review on the surgical management and outcomes of cholesteatomas of the petrous apex in the pediatric population. Outcomes investigated include presenting symptoms, initial imaging ordered, major complications (including reoperations), mean age of patient at diagnosis and surgical intervention, type of surgical intervention used, extent of resection, and average length of follow-up. In addition, we include a case of a 17-year-old patient presenting with a cholesteatoma of the external auditory canal with mastoid involvement [
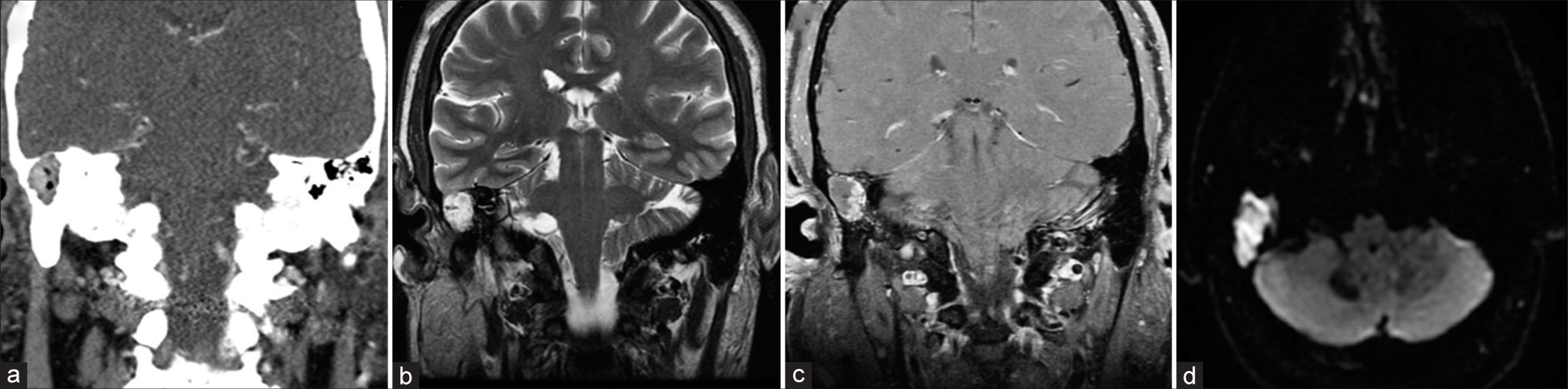
Figure 2:
A case of a 17-year-old patient with a delayed presentation of a cholesteatoma after initially presenting as a trauma from a motor vehicle accident and right sided otorrhea. (a) Computed tomography scan demonstrated 2 cm mass in the right medial aspect of the external auditory canal involving the right mastoid consistent with a cholesteatoma. (b-d) Magnetic resonance imaging of the lesion demonstrating, (b) T2 signal hyperintensity, and (c) mild irregular peripheral enhancement on T1 sequences with contrast. (d) DWI sequences demonstrating avid diffusion restriction, a characteristic feature of cholesteatomas.
Etiology
Cholesteatomas represent one of the most common pathologies of the petrous apex.[
Symptomatology
As epidermoid cysts of the petrous portion of the temporal bone, congenital petrous bone cholesteatomas may initially be asymptomatic if they extend in the direction of the nonpetrous portion of the temporal bone, leaving the tympanic membrane unscathed.[
To categorize facial nerve deficits, clinicians can use the House–Brackmann grading system for facial nerve function. Furthermore, pure-tone audiogram (PTA) can be performed to assess for hearing loss, and speech discrimination testing can screen for changes in speech recognition thresholds at specific decibels (dB) of sound. Interestingly, although most patients with petrous bone cholesteatomas will present with at least one of the aforementioned symptoms, hearing will not always be affected. For example, in their retrospective case series of eight patients, Orhan et al. reported that of eight patients who underwent endoscope-assisted surgery for petrous bone cholesteatoma, four exhibited normal-range hearing on preoperative PTA.[
Diagnosis
In patients presenting with suspicion for cholesteatoma, choice of imaging can be critical for accurate and timely diagnosis. The standard approach for detection involves MRI non-echo-planar DWI as well as high-resolution CT scan.[
In their case illustration, MacKeith et al. highlight the importance of choice of imaging in diagnosing and planning surgery for cholesteatoma.[
In the case of recurrent aseptic meningitis reported by MacKeith et al., lumbar puncture for CSF analysis before initiation of antibiotics was instrumental in directing subsequent choice of imaging.[
Surgical approaches
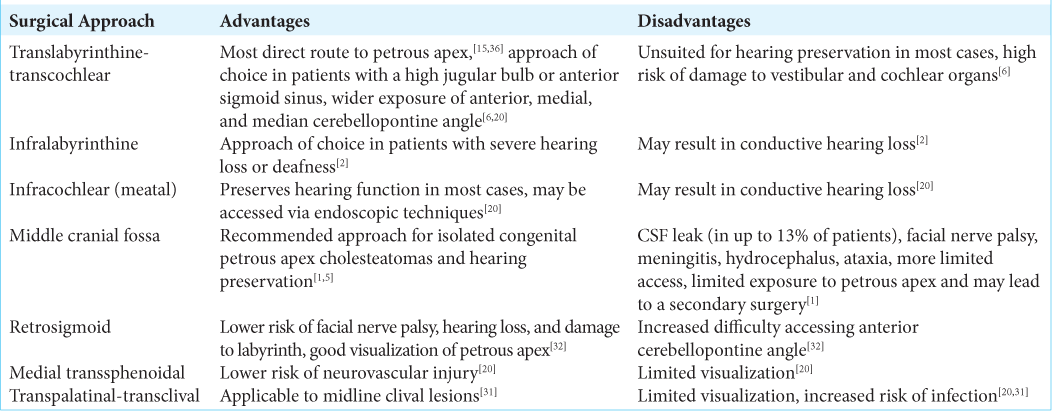
Surgical approaches for petrous apex cholesteatomas that have been previously described include subtotal petrosectomy, translabyrinthine, infralabyrinthine, supralabyrinthine, and middle cranial fossa techniques. In general, the most important aspect of the surgical approach is that it is optimized to the specific location of the cholesteatoma and overall patient at hand, including their anatomical variation.
Each of the surgical approaches offers a unique set of benefits, and some can predispose to complications more than others [
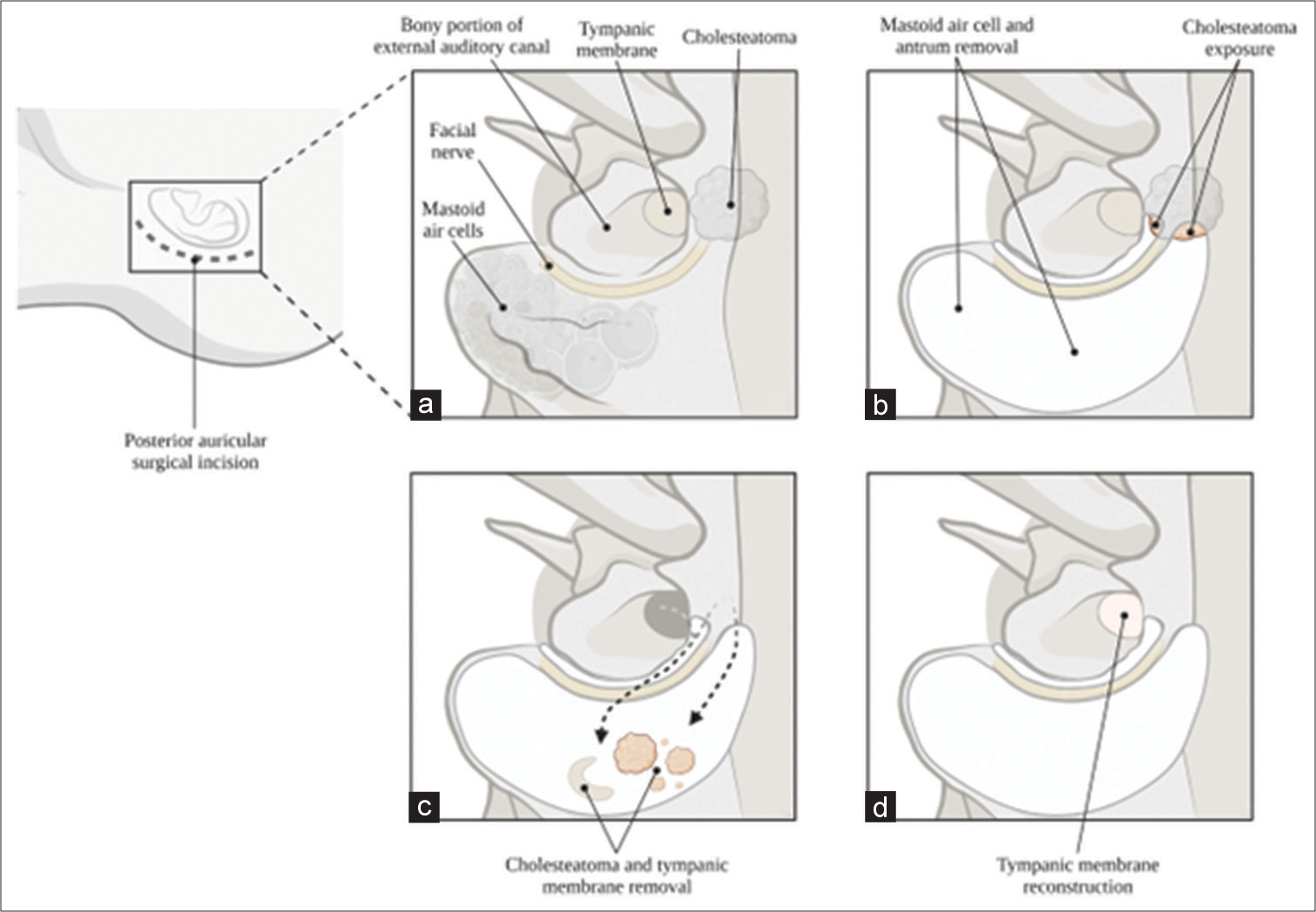
Figure 3:
Illustration of the canal-wall up procedure for removal of cholesteatomas of the petrous apex. (a) Orientation of anatomical landmarks relative to the cholesteatoma. (b) Removal of mastoid air cell and antrum for exposure of cholesteatoma. (c) Removal of cholesteatoma and tympanic membrane. (d) After removal, reconstruction of tympanic membrane.
Limitations and future perspectives
In this study, there were several limitations. Due to the exclusion of articles that were not published in English and did not include full texts, a publication bias could be present. Our results could over-suggest the utilization of the CWU approach to resection and underemphasize the utilization of the other surgical approaches mentioned. A low level of evidence is present due to the paucity of the literature available regarding this pathology in the pediatric population at this anatomical location. Future directions aim to establish a gold-standard approach to cholesteatoma resection at the petrous apex in pediatric patients and investigate risk factors for recurrence.
CONCLUSION
Cholesteatomas of the petrous apex are rare middle ear tumors that can appear in pediatric patients and, when untreated, can lead to facial nerve palsy and conductive hearing loss, along with higher susceptibility to recurrence in comparison to adults. Close follow-up after resection with MRI surveillance, in addition to a strong physical exam, is necessary to ensure proper management postoperatively. A comprehensive and tailored plan must be ensured for each patient to achieve desirable outcomes in pediatric patients with cholesteatomas of the petrous apex.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Atlas MD, Moffat DA, Hardy DG. Petrous apex cholesteatoma: Diagnostic and treatment dilemmas. Laryngoscope. 1992. 102: 1363-8
2. Bruchhage KL, Wollenberg B, Leichtle A. Transsphenoidal and infralabyrinthine approach of the petrous apex cholesterol granuloma. Eur Arch Otorhinolaryngol. 2017. 274: 2749-56
3. Casazza G, Carlson ML, Shelton C, Gurgel RK. The mediallyinvasive cholesteatoma: An aggressive subtype of a common pathology. Ann Otol Rhinol Laryngol. 2021. 130: 38-46
4. Chapman PR, Shah R, Curé JK, Bag AK. Petrous apex lesions: Pictorial review. AJR Am J Roentgenol. 2011. 196: WS26-37 Quiz S40-3
5. Corrales CE, Blevins NH. Imaging for evaluation of cholesteatoma: Current concepts and future directions. Curr Opin Otolaryngol Head Neck Surg. 2013. 21: 461-7
6. Cui H, Zhou CF, Bao YH, Wang MS, Wang Y. Extended suboccipital retrosigmoid surgical approach is effective for resection of petrous apex meningioma. J Craniofac Surg. 2016. 27: e429-33
7. De Foer B, Vercruysse JP, Bernaerts A, Deckers F, Pouillon M, Somers T. Detection of postoperative residual cholesteatoma with non-echo-planar diffusion-weighted magnetic resonance imaging. Otol Neurotol. 2008. 29: 513-7
8. De Foer B, Vercruysse JP, Bernaerts A, Maes J, Deckers F, Michiels J. The value of single-shot turbo spin-echo diffusion-weighted MR imaging in the detection of middle ear cholesteatoma. Neuroradiology. 2007. 49: 841-8
9. De Foer B, Vercruysse JP, Pilet B, Michiels J, Vertriest R, Pouillon M. Single-shot, turbo spin-echo, diffusion-weighted imaging versus spin-echo-planar, diffusion-weighted imaging in the detection of acquired middle ear cholesteatoma. AJNR Am J Neuroradiol. 2006. 27: 1480-2
10. Dhepnorrarat RC, Wood B, Rajan GP. Postoperative non-echo-planar diffusion-weighted magnetic resonance imaging changes after cholesteatoma surgery: Implications for cholesteatoma screening. Otol Neurotol. 2009. 30: 54-8
11. Fontes LA, Moreira FC, Menezes AS, Costa IE, Azevedo C, Sá Breda M. Is pediatric cholesteatoma more aggressive in children than in adults? A comparative study using the EAONO/JOS classification. Int J Pediatr Otorhinolaryngol. 2020. 138: 110170
12. Grauvogel J, Scheiwe C, Masalha W, Grauvogel T, Kaminsky J, Vasilikos I. Piezosurgery, neuroendoscopy, and neuronavigation-assisted intracranial approach for removal of a recurrent petrous apex cholesteatoma: Technical note. J Neurosurg Pediatr. 2018. 21: 322-8
13. Grinblat G, Prasad SC, Fulcheri A, Laus M, Russo A, Sanna M. Lateral skull base surgery in a pediatric population: A 25-year experience in a referral skull base center. Int J Pediatr Otorhinolaryngol. 2017. 94: 70-5
14. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008. 336: 924-6
15. House WF, De la Cruz A, Hitselberger WE. Surgery of the skull base: Transcochlear approach to the petrous apex and clivus. Otolaryngology. 1978. 86: ORL-770-9
16. James AL. Cholesteatoma in children: Surgical technique, hearing rehabilitation and surveillance. Curr Otorhinolaryngol Rep. 2018. 6: 82-91
17. Kanzara T, Virk JS, Chawda S, Owa AO. Wholly endoscopic permeatal removal of a petrous apex cholesteatoma. Case Rep Otolaryngol. 2014. 2014: 184230
18. Kuo CL, Shiao AS, Yung M, Sakagami M, Sudhoff H, Wang CH. Updates and knowledge gaps in cholesteatoma research. Biomed Res Int. 2015. 2015: 854024
19. Lehmann P, Saliou G, Brochart C, Page C, Deschepper B, Vallée JN. 3T MR imaging of postoperative recurrent middle ear cholesteatomas: Value of periodically rotated overlapping parallel lines with enhanced reconstruction diffusion-weighted MR imaging. AJNR Am J Neuroradiol. 2009. 30: 423-7
20. Li KL, Agarwal V, Moskowitz HS, Abuzeid WM. Surgical approaches to the petrous apex. World J Otorhinolaryngol Head Neck Surg. 2020. 6: 106-14
21. Louw L. Acquired cholesteatoma pathogenesis: Stepwise explanations. J Laryngol Otol. 2010. 124: 587-93
22. MacKeith SA, Soledad-Juarez M, Tiberti L, Orfila D. Recurrent aseptic meningitis as a rare but important presentation of congenital petrous apex cholesteatoma: The value of appropriate imaging. BMJ Case Rep. 2014. 2014: bcr2013010390
23. McGuire JK, Wasl H, Harris T, Copley GJ, Fagan JJ. Management of pediatric cholesteatoma based on presentations, complications, and outcomes. Int J Pediatr Otorhinolaryngol. 2016. 80: 69-73
24. Moffat D, Jones S, Smith W. Petrous temporal bone cholesteatoma: A new classification and long-term surgical outcomes. Skull Base. 2008. 18: 107-15
25. Muckle RP, De la Cruz A, Lo WM. Petrous apex lesions. Am J Otol. 1998. 19: 219-25
26. Orhan KS, Çelik M, Polat B, Aydemir L, Aydoseli A, Sencer A. Endoscope-assisted surgery for petrous bone cholesteatoma with hearing preservation. J Int Adv Otol. 2019. 15: 391-5
27. Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ. 2021. 372: n160
28. Piras G, Sykopetrites V, Taibah A, Russo A, Caruso A, Grinblat G. Long term outcomes of canal wall up and canal wall down tympanomastoidectomies in pediatric cholesteatoma. Int J Pediatr Otorhinolaryngol. 2021. 150: 110887
29. Preciado DA. Biology of cholesteatoma: Special considerations in pediatric patients. Int J Pediatr Otorhinolaryngol. 2012. 76: 319-21
30. Profant M, Steno J. Petrous apex cholesteatoma. Acta Otolaryngol. 2000. 120: 164-7
31. Pyle GM, Wiet RJ. Petrous apex cholesteatoma: Exteriorization vs. Subtotal petrosectomy with obliteration. Skull Base Surg. 1991. 1: 97-105
32. Rubio RR, Xie W, Vigo V, Lee A, Tomasi OS, El-Sayed I. Immersive surgical anatomy of the retrosigmoid approach. Cureus. 2021. 13: e16068
33. Sanna M, Zini C, Gamoletti R, Frau N, Taibah AK, Russo A. Petrous bone cholesteatoma. Skull Base Surg. 1993. 3: 201-13
34. Shihada R, Brodsky A, Luntz M. Giant cholesteatoma of the temporal bone. Isr Med Assoc J. 2006. 8: 718-9
35. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016. 355: i4919
36. Sudhoff H, Klingebiel R, Scholtz LU, Todt I. Translabyrinthine petrous apex cholesteatoma surgery with hearing preservation. Case Rep Otolaryngol. 2021. 2021: 5541703
37. Takahashi K, Morita Y, Aizawa N, Ogi M, Nonomura Y, Kitazawa M. Patient-specific 3D-printed model-assisted supracochlear approach to the petrous apex. Otol Neurotol. 2020. 41: e1041-5
38. Van Dinther JJ, Vercruysse JP, De Foer B, Somers T, Zarowski A, Casselman J. Subarcuate supralabyrinthine approach for supralabyrinthine petrosal cholesteatoma. Ann Otol Rhinol Laryngol. 2010. 119: 42-6
39. Zulkifli MF, Saim LB. Congenital cholestratoma of petrous apex: A case report. J Otolaryngol Rhinol. 2019. 5: 58