- Department of Neurosurgery, Faculty of Medicine, Ain Shams University, Cairo,
- Department of Pediatrics, Faculty of Medicine, Minia University, El Minia, Egypt.
Correspondence Address:
Hesham Radwan, Department of Neurosurgery, Faculty of Medicine, Ain Shams University, Cairo, Egypt.
DOI:10.25259/SNI_7_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ahmed Darwish1, Hesham Radwan1, Zeiad Fayed1, Samir M. Mounir2, Salah Hamada1. Surgical nuances in corpus callosotomy as a palliative epilepsy surgery. 25-Mar-2022;13:110
How to cite this URL: Ahmed Darwish1, Hesham Radwan1, Zeiad Fayed1, Samir M. Mounir2, Salah Hamada1. Surgical nuances in corpus callosotomy as a palliative epilepsy surgery. 25-Mar-2022;13:110. Available from: https://surgicalneurologyint.com/surgicalint-articles/11478/
Abstract
Background: Corpus callosotomy is a well-established palliative procedure in selected patients with drug resistant epilepsy (DRE). It has a beneficial role in ameliorating generalized seizures mainly drop attacks. Here, we present some technical tips for performing callosotomy depending on the anatomical basis, to minimize craniotomy size and guard against inadvertently entering the lateral ventricles.
Methods: This study was a retrospective review of patients who received corpus callosotomy at our institute as a palliative epilepsy surgery. We present our experience and surgical tips with the extraventricular technique of corpus callosotomy with comparison of surgery-related complications and operative time between extraventricular and conventional techniques in selected patients with DRE.
Results: Our study included 34 patients. First group of patients included 14 patients who received conventional approach, while the extraventricular approach was done in 20 patients. Extraventricular approach showed significantly lower wound complications rate of 10% compared to 78% in intraventricular approach (P P
Conclusion: The cleft of the septum pellucidum offers a natural pursuit to section corpus callosum strictly midline and completely extraventricular in well selected patients of DRE candidate for callosotomy. Performing corpus callosotomy in extraventricular approach provided better patients outcomes regarding surgery and wound-related complications when compared to conventional approach.
Keywords: Drug resistant epilepsy surgery, Epilepsy surgery, Extraventricular corpus callosotomy, Surgery related complications of corpus callosotomy
INTRODUCTION
Corpus callosotomy is widely accepted palliative procedure for patients with drug resistant generalized epilepsy drug resistant epilepsy (DRE) whom their main burden is frequent drop attacks if there is no identifiable focus. After observing a reduction in generalized seizure activity in two patients with callosal tumors and two cases of callosal vascular insult, Van Wagenen and Herren introduced corpus callosotomy in 1940 as a treatment for reducing generalized convulsive seizures.[
Corpus callosum is composed of over 200 million myelinated axons and considered the largest commissural white matter band of fibers connecting both hemispheres thus playing a critical role in generalization of epilepsy. The septum pellucidum (SP) is a two layered structure in a strictly midline position, located ventral to the body of corpus callosum separating between the two lateral ventricles extending from the rostrum anteriorly to the splenium posteriorly.[
The surgical technique of corpus callosotomy has been well described in the literature as a palliative epilepsy surgery in patients with DRE,[
In our early experience of the corpus callosotomy procedure, we performed the conventional technique adopted for callosotomy.[
MATERIALS AND METHODS
This was a retrospective analysis of all patients with drug resistant generalized epilepsy who underwent callosotomy in the period from 2016 to 2020 at our institute. We divided the patients into two groups according to the surgical technique used for callosotomy. First group of patients included those done through the conventional technique.[
The choice of the extent of callosotomy was totally dependent on preoperative clinical and radiological evaluation performed by the integrated efforts of a multidisciplinary epilepsy team to determine the eligibility and fitness of the patient for corpus callosotomy.
Preoperative MRI brain was thoroughly studied especially the sagittal MRI view to determine
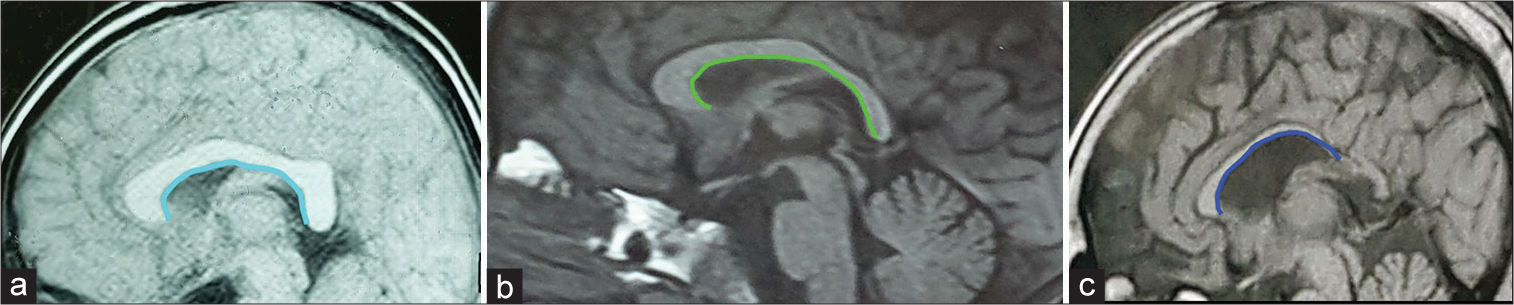
The shape of corpus callosum which in our study population was as either: (a) straighten, (b) lazy c-shaped, or (c) exaggerated c-shaped that helps in defining the relative depth between the body of corpus callosum and genu or the splenium of corpus callosum, thus anticipating the degree of sloping while performing callosotomy [ The thickness of the genu, the body and splenium of corpus callosum, with focus on course and relations of the pericallosal arteries along the corpus callosum and the possible position of cortical bridging veins entering the superior sagittal sinus (SSS), to avoid injury of underlying vascular elements Relation of the coronal suture to the body of the corpus callosum to design the craniotomy flap, we usually perform a craniotomy flap 3 cm anterior to the coronal suture to perform anterior corpus callosotomy (ACC) and a 5 cm craniotomy flap centered on coronal suture is sufficient for complete corpus callosotomy (CCC), while we use a craniotomy flap 4 cm with 3 cm posterior to coronal suture for posterior corpus callosotomy.
Operative technique
The patient is placed in supine position, head is neutrally placed on head rest, a craniotomy bone flap is designed and performed according to the extent of callosotomy planned. A c-shaped dural opening based on SSS is done in a meticulous fashion to safeguard against injury of the cortical draining veins entering the SSS. The frequent presence of posteriorly placed critical veins usually limits the opening of the dura beyond the level of coronal suture.
Using sharp microdissection, both hemispheres are separated in a step-wise approach along the falx cerebri helped by CSF suction from pericallosal cistern then identify the landmarks.
Callosomarginal artery within its sulcus, pericallosal arteries resting on the corpus callosum and the glistening white CC itself.
Then, dissection is made along the length of the corpus callosum in between the two pericallosal arteries starting anteriorly from the genu backward as the diameters of both arteries permit less difficult dissection besides the acute sink of both arteries around the corpus callosum edge, marking an important intraoperative landmark for the genu of CC. Identifying this landmark is the key to avoid entering the lateral ventricles.
The space in between the arteries is maintained by small cottonoid anteriorly and posteriorly.
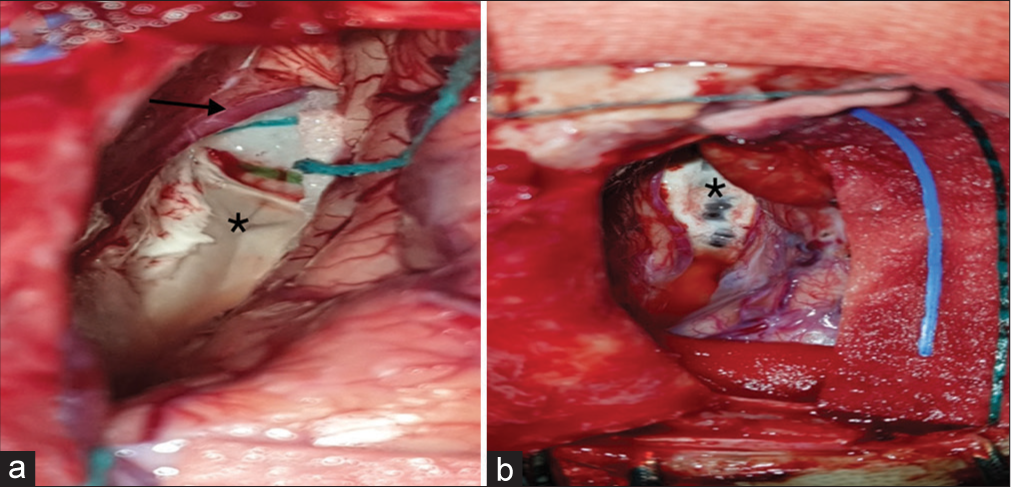
Division of the corpus callosum is initiated using the blades of bipolar cautery helped by Rhoton 6 suction till the cleft between the two layers of the SP is reached, using nine Rhoton dissector, this cleft is dissected and exposed anteriorly and posteriorly thus dividing the corpus callosum strictly at the midline without entering the ventricles [
Figure 2:
Intraoperative images showing differences in both techniques. (a) Extraventricular technique with images showing the cleft of the septum pellucidum. Arrow towards pericallosal artery and asterix at the cleft of corpus callosum. (b) Conventional technique intraoperative image with asterix at the exposed ependyma of the ventricles at risk for unintended disruption.
Following the SP, cleft anteriorly leads us to the genu which is thickened anterior part of the corpus callosum, continuous dissection and subpial suction through a genu helped by preoperative anticipation of its estimated thickness, then the mission is accomplished when both pericallosal arteries are back in the scene within the suprasellar cistern, the same procedure is applied to the splenium posteriorly with the veins in the quadrigeminal cistern to be encountered at the end of dissection.
The main aim of the analysis is to compare postoperative surgery-related complications between patients who underwent callosotomy using the extraventricular approach with those for whom the conventional approach was used.
Statistical analysis
The collected data were revised, coded, tabulated, and introduced to a PC using Statistical Package for the Social Science 20. Continuous data were summarized as mean and standard deviation while categorical data were presented as counts and percentages. For comparison between outcomes of the intraventricular versus extra ventricular approaches, Chi-square test and Fisher exact test were used for categorical data and t-test for normally distributed continuous data.
RESULTS
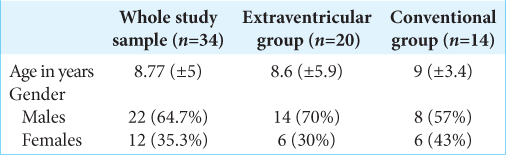
In the defined period from 2016 to 2020, we identified 34 cases with drug resistant epilepsy who underwent callosotomy and were included in the analysis. Fourteen out of the 34 patients were in the first group, in which corpus callosotomy was done through the conventional technique. The second group included 20 patients, in which extraventricular technique was done.
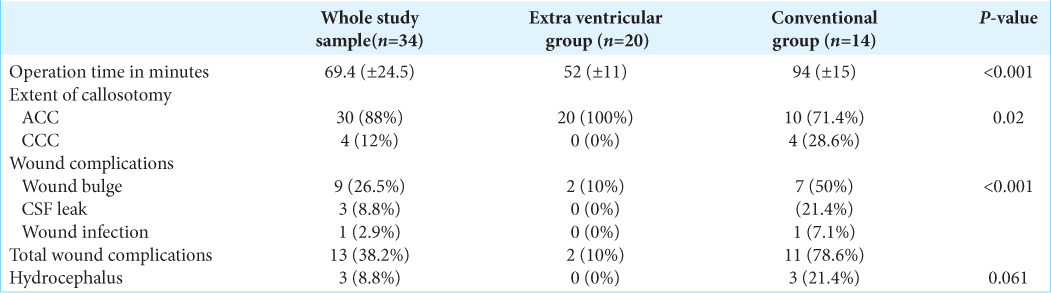
The study population included 22 males (64.7%) and 12 (35.3%) females with a mean age at the time of surgery of 8.77 years (±5). Most of our patients (30 patients) representing 88% of the sample had ACC, while only 4 patients (1%) underwent CCC with mean operation time of 69.4 min (±24.52) for all patients [
In analysis of surgery-related complications in our study population, we had postoperative wound complications in 13 patients (38.2%). These wound complications were in the form of wound bulge in 26.5% of all patients, while CSF leakage was in 8.8% and wound infection in 2.9% patients. Only 3 patients (8.8%) required CSF diversion due to the development of postoperative hydrocephalus [
According to the analytical statistics applying student t-test, Chi-square test, and Fisher’s exact test, there was a highly significant difference in the rate of surgery-related wound complications between both techniques (extraventricular versus conventional technique) being significantly lower in the extraventricular one. Only two patients in the extraventricular groups versus 11 patients for the conventional technique developed surgery-related wound complications, either wound bulge, CSF leakage, or infection (P < 0.001). Of note, 9/14 patients in the conventional group experienced unintended lateral ventricular wall breech during the procedure. Planned extent of corpus callosotomy either anterior or complete was amenable in all our cases in both groups [
DISCUSSION
We described a technique which involves section of the corpus callosum with decreased probability of ventricular wall breach guided by the anatomical fact that the SP is composed of two layers which is incompletely fused dorsally below the corpus callosum regardless of the presence or absence of the cavum SP. Embryologically, SP is developed from two anatomical layers, which are fused starting around 20 weeks of gestation. Incomplete fusion of SP (sparring a small dorsal cleft) usually develops at the age of 6 months. Thus, the dorsal most aspect of the SP offers a small cleft that acts as a railway to be followed for safe section of the corpus callosum.[
This anatomical fact gives the surgeon good chance to be strictly at the midline cutting the corpus callosum at its thickest part without paramedian breaching the thinned out ependymal lining, the cutting is advanced anteriorly till the line ending at the genu, and posteriorly at the splenium if needed.
Both intra and extraventricular techniques were perfectly efficient in carrying on callosotomy. However, considering results of our study, extraventricular technique had superior advantage of significantly reducing surgery-related complications with callosotomy such as wound bulge, CSF leakage, or wound infection. Our results showed significantly lower rate of surgery related wound complications in patients received callosotomy through the extraventricular technique, 10% compared to 78% in whom the conventional technique was performed.
Other studies reported surgery-related complications such as epidural hematoma, subdural hematoma, meningitis, and external hydrocephalus in 7–20% in patients who received open corpus callosotomy.[
Since the corpus callosotomy technique was first described in the literature, there was an emphasis to avoid disruption of the ependymal lining of the lateral ventricle, which is not always easily amenable.[
It is reported in the literature that the thickness of the corpus callosum is increased in patients with generalized epilepsy compared to normal population,[
With improvement of our learning curve in performing callosotomy through the extraventricular technique, it was found to be less time-consuming. Our results showed that the mean operative time for the extraventricular operative technique was 52 min compared to 94 min for that reported in our early cases of the intraventricular technique. This decrease in the operative time can be related to gaining more experience through our learning curve. Other studies of open, endoscopic, and laser interstitial callosotomy reported mean operative time 1–2½ hours.[
In our series, we encountered no mortality or major morbidity related to any of the two techniques except for three patients in the intraventricular group who suffered from delayed hydrocephalus requiring CSF diversion. Major surgery-related complication was previously reported in 1.5– 2% of patients who received open surgical callosotomy.[
CONCLUSION
The cleft of the SP offers a natural pursuit to section corpus callosum strictly midline and completely extraventricular in well selected patients of DRE candidate for callosotomy. Performing corpus callosotomy with the extraventricular approach provided better patients outcomes regarding surgery and wound-related complications when compared to conventional approach.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Asadi-Pooya AA, Sharan A, Nei M, Sperling MR. Corpus callosotomy. Epilepsy Behav. 2008. 13: 271-8
2. Baumgartner JE, Ajmal FQ.editors. Palliative surgical techniques (VNS, Callosotomy). Textbook of Pediatric Neurosurgery. Berlin, Germany: Springer; 2020. p. 2203-19
3. Conlon P, Trimble MR. A study of the corpus callosum in epilepsy using magnetic resonance imaging. Epilepsy Res. 1988. 2: 122-6
4. Dwivedi R, Ramanujam B, Chandra PS, Sapra S, Gulati S, Kalaivani M. Surgery for Drug-Resistant Epilepsy in Children. N Engl J Med. 2017. 377: 1639-47
5. Goldstein A, Covington BP, Mahabadi N, Mesfin FB.editors. Neuroanatomy, Corpus Callosum. Treasure Island, FL: StatPearls; 2021. p.
6. Jea A, Vachhrajani S, Johnson KK, Rutka JT. Corpus callosotomy in children with intractable epilepsy using frameless stereotactic neuronavigation: 12-year experience at The Hospital for Sick Children in Toronto. Neurosurg Focus. 2008. 25: E7
7. Joseph JR, Viswanathan A, Yoshor D. Extraventricular corpus callosotomy. J Neurosurg. 2011. 114: 1698-700
8. Kasasbeh AS, Smyth MD, Steger-May K, Jalilian L, Bertrand M, Limbrick DD. Outcomes after anterior or complete corpus callosotomy in children. Neurosurgery. 2014. 74: 17-28
9. Luders E, Thompson PM, Toga AW. The development of the corpus callosum in the healthy human brain. J Neurosci. 2010. 30: 10985
10. Maehara T, Shimizu H. Surgical outcome of corpus callosotomy in patients with drop attacks. Epilepsia. 2001. 42: 67-71
11. Mamelak AN, Barbaro NM, Walker JA, Laxer KD. Corpus callosotomy: A quantitative study of the extent of resection, seizure control, and neuropsychological outcome. J Neurosurg. 1993. 79: 688-95
12. Quiñones-Hinojosa A, Schmidek HH.editors. Schmidek and Sweet Operative Neurosurgical Techniques : Indications, Methods, and Results. Netherlands: Elsevier; 2021. p.
13. Roland JL, Akbari SH, Salehi A, Smyth MD. Corpus callosotomy performed with laser interstitial thermal therapy. J Neurosurg. 2021. 134: 314-22
14. Scheuer C, Boot E, Carse N, Clardy A, Gallagher J, Heck S.editors. Corpus Callosotomy and Multiple Subpial Transection. Physical Education and Sport for Children and Youth with Special Needs Researches-best Practices-situation. 2015. p. 343-54
15. Snell RS.editors. Clinical Neuroanatomy. Netherlands: Wolters Kluwer; 2010. p.
16. Sunaga S, Shimizu H, Sugano H. Long-term follow-up of seizure outcomes after corpus callosotomy. Seizure. 2009. 18: 124-8
17. Tanriverdi T, Olivier A, Poulin N, Andermann F, Dubeau F. Long-term seizure outcome after corpus callosotomy: A retrospective analysis of 95 patients. J Neurosurg. 2009. 110: 332-42
18. Turanli G, Yalnizoğlu D, Genç-Açikgöz D, Akalan N, Topçu M. Outcome and long term follow-up after corpus callosotomy in childhood onset intractable epilepsy. Childs Nerv Syst. 2006. 22: 1322-7
19. Uda T, Kunihiro N, Umaba R, Koh S, Kawashima T, Ikeda S. Surgical aspects of corpus callosotomy. Brain Sci. 2021. 11: 1608
20. Van Wagenen WP, Herren RY. Surgical division of commisissural pathways in the corpus callosum: Relation to spread of an epileptic attack. J Neurv Mental Dis. 1940. 44: 740-59
21. Vannucci RC, Barron TF, Vannucci SJ. Development of the Corpus Callosum: An MRI Study. Dev Neurosci. 2017. 39: 97-106
22. Winn HR.editors. Youmans and Winn Neurological Surgery. Netherlands: Elsevier; 2017. p. 8256-64
23. Wong TT, Kwan SY, Chang KP, Hsiu-Mei W, Yang TF, Chen YS. Corpus callosotomy in children. Childs Nerv Syst. 2006. 22: 999-1011