- Department of Neurosurgery, Osaka Medical and Pharmaceutical University, Tskatsuki City, Osaka, Japan.
Correspondence Address:
Ryokichi Yagi, Department of Neurosurgery, Osaka Medical and Pharmaceutical University, Tskatsuki City, Osaka, Japan.
DOI:10.25259/SNI_1056_2021
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Takumi Hoshimaru, Ryokichi Yagi, Shinji Kawabata, Masahiko Wanibuchi. Surgical strategy for tuberculous brain abscess with dural infiltration: A case report. 05-Jan-2022;13:4
How to cite this URL: Takumi Hoshimaru, Ryokichi Yagi, Shinji Kawabata, Masahiko Wanibuchi. Surgical strategy for tuberculous brain abscess with dural infiltration: A case report. 05-Jan-2022;13:4. Available from: https://surgicalneurologyint.com/surgicalint-articles/11329/
Abstract
Background: Tuberculosis is one of the top 10 leading causes of death worldwide. Although tuberculous central complications account for 1% of all tuberculosis patients, there are many cases of medical resistance; and early surgical treatment is required for brain abscess. Reports on tuberculous brain abscesses with dural infiltration are rare, and there are no reports on surgical treatment methods.
Case Description: An 81-year-old man was presented with the right arm paresis. His recent medical history included a 6-month course of immunosuppressants, and steroids prescribed for ulcerative colitis, and four antituberculosis drugs had been started 2 months before for relapse of pulmonary tuberculosis at an early age. Head T1-weighted magnetic resonance imaging with administration of gadolinium showed two ring-enhanced lesions in the left precentral gyrus and continuous with the dura mater. Surgery was performed and he was pathologically diagnosed with a tuberculous brain abscess. Since the pathological diagnosis revealed dura mater invasion, we removed the dura mater and reconstructed by periosteum. After the surgery, the symptoms gradually improved, and the abscess and edema improved when viewed on the image. Despite the administration of steroids for ulcerative colitis without antituberculosis drugs, no recurrence was observed for 1 year. Recurrence of tuberculous brain abscess is a major problem in immunosuppressed patients, but it is considered that the relapse could be prevented by removing the dural infiltration.
Conclusion: In cases of tuberculous brain abscess with dural infiltration, it is considered that the recurrence can be prevented even in an immunosuppressed state by removing the dura.
Keywords: Dural infiltration, Immunosuppressed patients, Surgical technique, Tuberculous brain abscesses, Tuberculous central complications
INTRODUCTION
About 10 million new cases of tuberculosis occur worldwide every year, thereby making it one of the top 10 leading causes of death.[
CASE REPORT
Case presentation
An 81-year-old man was presented with the right arm paresis. He had a medical history of pulmonary tuberculosis when he was in junior high school. His recent medical history included a 6-month course of immunosuppressants, and steroids prescribed for ulcerative colitis, and he was taking steroids until 2 weeks before coming hospital. In addition, for the past 3 months, he has been taking four antituberculosis drugs (isoniazid 300 mg, ethambutol hydrochloride 750 mg, pyrazinamide 1.2 g, and rifampicin 450 mg) for relapse of latent pulmonary tuberculosis. During the same time, cervical lymph node biopsy revealed papillary thyroid cancer (Stage IIB, cervical lymph node metastasis), and surgical treatment was considered.
Symptoms were difficulty in writing and weakness in his right-hand grip, and when it became difficult to raise his right upper limb, he presented to the hospital.
On admission, his height was 171 cm, weight was 62.8 kg, blood pressure was 161/89 mmHg, body temperature was 36.5°C, and oxygen saturation was 98% (room air). His consciousness was clear, and neurologic findings revealed right mouth ptosis, dysarthria, and right upper limb paresis (manual muscle test [MMT] Grade 4/5). Blood test results were leukocytes 3010/μL, hemoglobin 9.8 g/dL, platelets 183,000/μL, aspartate aminotransferase 29 U/L, alanine aminotransferase 20 U/L, blood urea nitrogen 17 mg/dL, serum creatinine 1.15 mg/dL, C-reactive protein 1.37 mg/dL, D-dimer 2.3 μg/m, carbohydrate antigen 19–9 51.3 U/mL (normal, <37.0 U/mL), cancer antigen 125 63.2 U/mL (normal, <35.0 U/mL), HIV antibody 0.05 S/CO(<1.00 S/CO), and HIV antigen/antibody 0.17 S/CO (<1.00 S/CO). The tuberculosis DNA-loop-mediated isothermal amplification (LAMP) test and sputum mycobacteria smear were positive. Cerebrospinal fluid test results were glucose 68 mg/dL, total protein 43.7 mg/dL, and cells 4/μL; cerebrospinal fluid culture was negative.
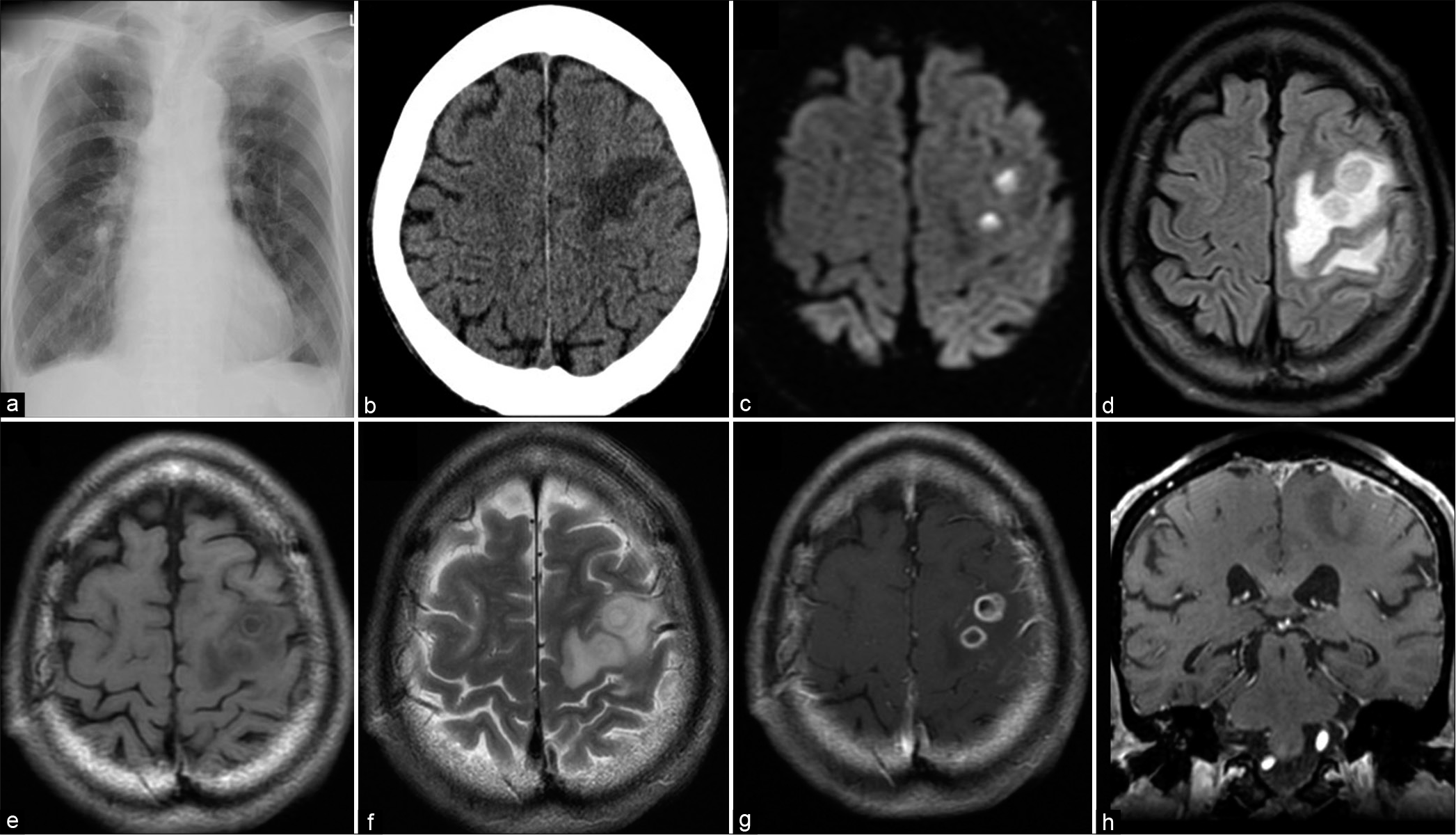
Chest radiography showed patchy shadows and the previous tuberculosis with calcification in the lower right lung lobe; however, no obvious exacerbation was observed after the start of antituberculosis drug [
Figure 1:
Chest X-ray shows mottled shadow and old tuberculosis with calcification in the right lower lung lobe (a). Computed tomography shows a low-density lesion in the left precentral gyrus (b). Magnetic resonance imaging shows the lesions high intensity on a diffusion-weighted image (c). Fluid-attenuated inversion recovery image shows high-intensity area around the lesions (d), low intensity on T1-weighted image (T1WI) (e), and high intensity on a T2WI (f). T1WI with administration of gadolinium shows two ring-enhanced lesions in the left precentral gyrus on axial image, and dural-enhanced lesion on coronal image (g and h).
Because the patient had a cancer and was taking immunosuppressive drugs and steroids, metastatic dural tumor and bacterial brain abscess were suspected, and surgery was considered for diagnosis and treatment. However, because the tuberculosis DNA-LAMP test and sputum acid-fast bacillus smear were positive, the risk of spreading tuberculosis infection during the perioperative period was considered, so conservative treatment was selected using antibiotic administration to address the first diagnosis considered, bacterial brain abscess.
Seventeen days after the start of the antibiotic, the right arm paralysis worsened to MMT 1/5, and the imaging findings showed no improvement for the contrast-enhanced lesions or peritumoral edema. At this point, the sputum acid-fast bacillus culture taken at admission was confirmed as negative, so the risk of perioperative infection spread was judged to be low, and craniotomy was performed on the hospital day 18.
Operation and pathological diagnosis
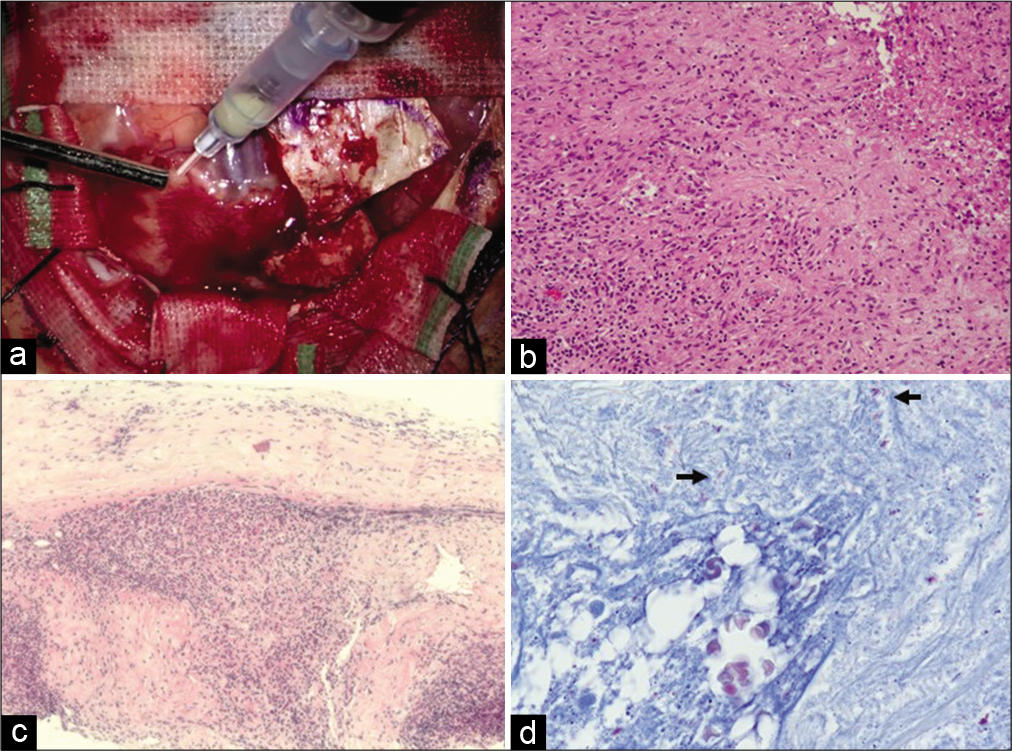
When the dura mater just above the contrast lesion was incised, the lesion was easy to peel off from dura, but there was a finding that part of the dura was infiltrated. The boundary with the normal brain was unclear and the adhesion was strong, making it difficult to peel the tumor off the brain. When the contents of the cyst were punctured and aspirated, a liquid suspected to be white turbid pus was collected [
Figure 2:
(a) This intraoperative photograph shows an abscess of a mass lesion. (b) Photomicrography of the dura mater shows granuloma with infiltration of inflammatory cells mainly composed of neutrophils and lymphocytes. (c) Granuloma formation and inflammatory cell infiltration were observed in the dura mater. (d) Arrows show the acid-fast bacilli in Ziehl–Neelsen stain.
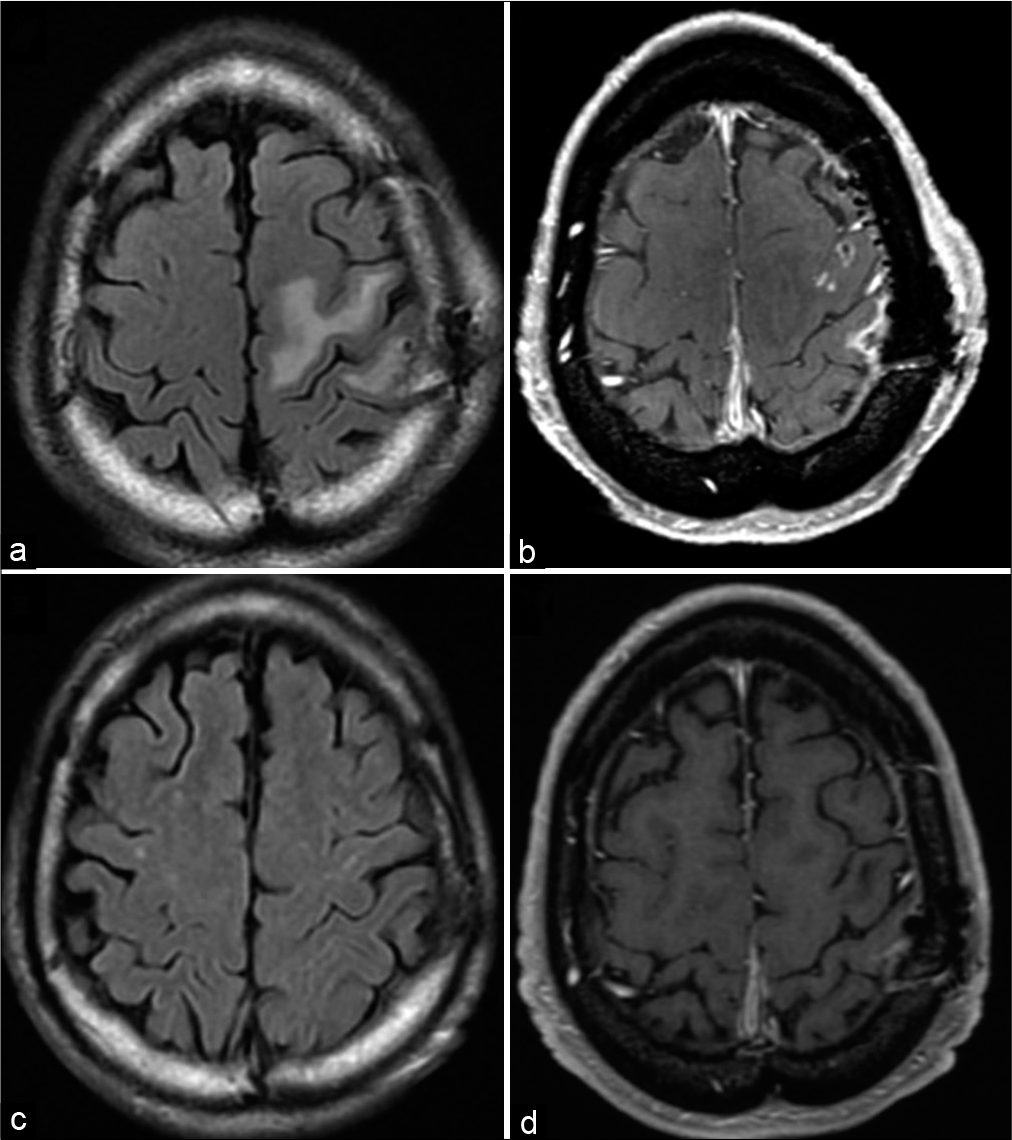
Postoperative course
The paralysis of the right upper arm gradually improved postoperatively and recovered to MMT 5/5 at postoperative week 2. Head T1WI MRI with gadolinium showed no residual enhanced lesion, and FLAIR showed reduction of the peritumoral high-intensity lesion [
DISCUSSION
In Japan, the number of newly diagnosed tuberculosis patients was 14,460 in 2019, and the number of newly diagnosed patients was 11.5 per 100,000 population. Although the incidence of tuberculosis has decreased in the past 20 years, it is still high compared with Western countries.[
Imaging features of tuberculous brain abscess include: (1) size ≥3 cm, (2) the presence of restricted diffusion, and (3) a thin and smooth mass wall. Multiple lesions are considered to suggest tuberculous brain abscess rather than intracranial tuberculoma, and more prominent peritumoral edema has been reported for tuberculous brain abscess than for intracranial tuberculoma.[
The Whitener diagnostic criteria for tuberculous brain abscess include: (1) presence of pus by microscopic findings, (2) denial of granulomatous changes such as giant cells and epithelioid cells by pathologic findings, and (3) proof of tubercle bacilli by culture test or stain with acid bacterium.[
One of the radiological differentiating features between tuberculous brain abscess and bacterial brain abscess is that the cyst wall is thicker in the tuberculous brain abscess than bacterial brain abscess, but in reality, there are few other characteristic differentiating features. It is often difficult to differentiate them radiologically.[
For the treatment of tuberculous brain abscess, it is effective to perform surgery and administer antituberculosis drugs simultaneously. Surgical treatment includes abscess aspiration, drainage, and abscess removal.[
CONCLUSION
The report presents a case of tuberculous brain abscess that was difficult to distinguish from bacterial brain abscess and metastatic dural tumor. The treatment strategy for tuberculous brain abscess has not been established, and it is necessary to consider the treatments from antituberculosis drugs and surgical interventions such as abscess aspiration, drainage, and abscess removal, depending on the case. In tuberculous brain abscess with dural infiltration, dural resection is considered necessary to prevent recurrence.
I have the patient’s consent to academic presentations and publications.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
I wish to thank Dr. Takuya Kanemitsu, Dr. Naokado Ikeda, Dr. Naosuke Nonoguchi, and Dr. Motomasa Furuse for advice on experimental design.
References
1. Belfquih H, Akhaddar A. Stereotactic aspiration in management of rare case of tuberculous cerebellar abscess. World Neurosurg. 2019. 129: 188-9
2. Gupta RK, Pandey R, Khan EM, Mittal P, Gujral RB, Chhabra DK. Intracranial tuberculomas: MRI signal intensity correlation with histopathology and localised proton spectroscopy. Magn Reson Imaging. 1993. 11: 443-9
3. Khatri GD, Krishnan V, Antil N, Saigal G. Magnetic resonance imaging spectrum of intracranial tubercular lesions: One disease, many faces. Pol J Radiol. 2018. 83: 524-35
4. Kumar R, Pandey CK, Bose N, Sahay S. Tuberculous brain abscess: Clinical presentation, pathophysiology and treatment (in children). Childs Nerv Syst. 2002. 18: 118-23
5. 6. Mohindra S, Savardekar A, Gupta R, Tripathi M, Rane S. Tuberculous brain abscesses in immunocompetent patients: A decade long experience with nine patients. Neurol India. 2016. 64: 66-74 7. Sahoo IK, Satapathy AK, John J. Multiple tubercular brain abscesses with obstructive hydrocephalus in an immunocompetent child: A case report. J Pediatr Neurosci. 2020. 15: 38-41 8. Shuangshoti S, Shuangshoti S. Tuberculous brain abscess: A case report with a review of the literature in English. Neuropathology. 1999. 19: 328-35 9. Tanaka M, Matsumoto S. Tuberculous brain abscess A case report. Neurol Med Chir (Tokyo). 1982. 22: 235-40 10. Whitener DR. Tuberculous brain abscess. Report of a case and review of the literature. Arch Neurol. 1978. 35: 148-55