- Department of Vascular, Burdenko Neurosurgical Center, Moscow, Russian Federation.
- Department of Neuroedoscopy, Burdenko Neurosurgical Center, Moscow, Russian Federation.
Correspondence Address:
Anton Konovalov, Department of Vascular, Burdenko Neurosurgical Center, Moscow, Russian Federation.
DOI:10.25259/SNI_843_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Anton Konovalov1, Oleg Sharipov2, Oleg Shekhtman1, Vadim Gadzhiagaev1, Pavel Kalinin2. Surgical treatment of ruptured right middle cerebral artery mycotic aneurysm and central nervous system aspergillosis: Clinical case and literature review. 08-Nov-2021;12:555
How to cite this URL: Anton Konovalov1, Oleg Sharipov2, Oleg Shekhtman1, Vadim Gadzhiagaev1, Pavel Kalinin2. Surgical treatment of ruptured right middle cerebral artery mycotic aneurysm and central nervous system aspergillosis: Clinical case and literature review. 08-Nov-2021;12:555. Available from: https://surgicalneurologyint.com/surgicalint-articles/11219/
Abstract
Background: Central nervous system (CNS) aspergillosis is more often met in patients with expressed immune suppression. Still, in 50% of cases of meningitis caused by Aspergillus spp., it is observed in patients without expressed immune suppression. The prognosis of CNS aspergillosis is unfavorable with the general rate of lethality around 70%.
Case Description: Clinical case of a 58-year-old man who developed an Aspergillus abscess in the chiasmosellar region and an associated mycotic aneurysm of the right middle cerebral artery (MCA) and intracerebral hemorrhage. Microsurgical clipping of the fusiform-ectatic aneurysm of the right MCA in the conditions of rupture was performed. An extra-intracranial micro anastomosis was formed on the right. An open biopsy of the neoplasm in the chiasmosellar region was made. The neoplasm was yellow and destroyed the bone plate of the skull base. Biopsy results: Mycotic lesion (aspergillosis). The analysis of surgical treatment for mycotic aneurysms in the acute period of hemorrhage in patients with aspergillosis revealed a high rate of lethality. The issue of the feasibility and effectiveness of complicated revascularization interventions in the patients with hemorrhage and aspergillosis remains unsolved.
Conclusion: The lack of generally accepted tactics of the treatment of this pathology requires further studies and systemic analysis. A high risk of the lethal outcome in patients with invasive mycotic infection and rupture of mycotic aneurysm highlight the importance of timely diagnostics and the beginning of antimycotic therapy. WThe issue of the evaluation of the revascularization methods effectiveness in patients after surgical treatment of a mycotic aneurysm associated with cerebral aspergillosis remains poor.
Keywords: Aspergillosis, Bypass, Ruptured aneurysm
INTRODUCTION
Central nervous system (CNS) aspergillosis is more often met in patients with expressed immune suppression. Still, in 50% of cases of meningitis caused by Aspergillus spp., it is observed in patients without expressed immune suppression.[
CLINICAL CASE
Patient X. 58 years old
The patient noticed a persistent decrease in the body weight by 8–10 kg within a month. He underwent examination for a decrease in the quality of vision (OD – 0.1, OS – 0). Brain magnetic resonance imaging (MRI) revealed a mass lesion in the chiasmosellar region [
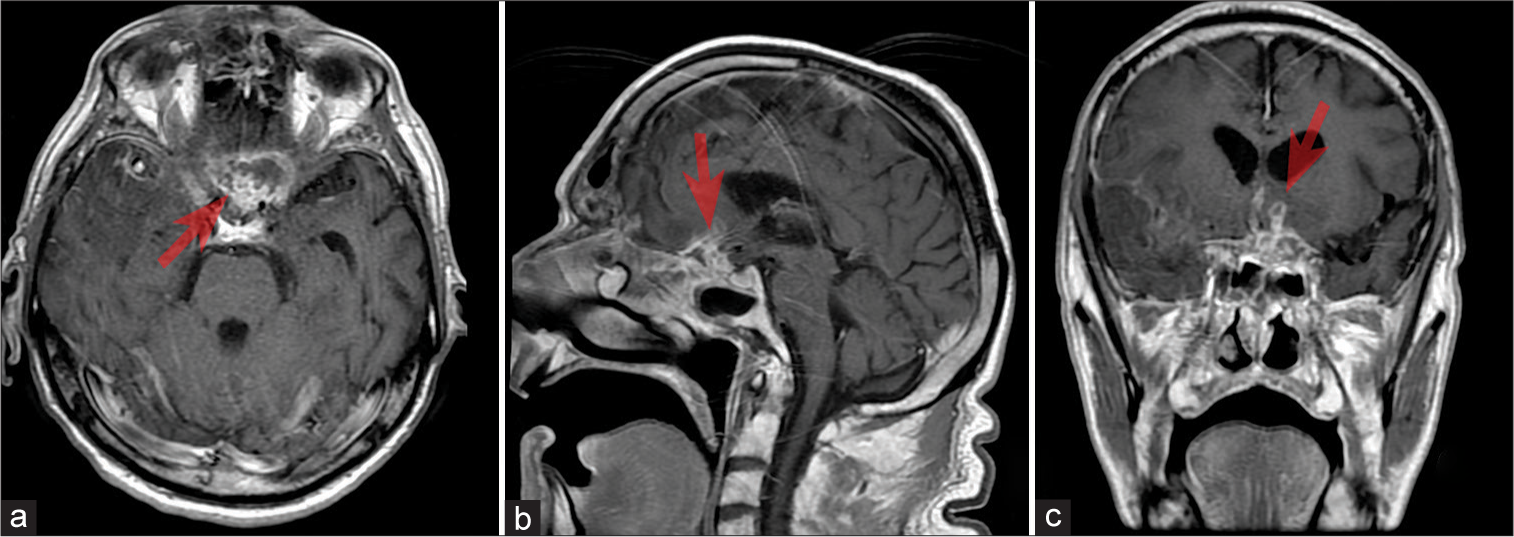
Figure 1:
(a-c) Axial, sagittal, and frontal magnetic resonance imaging projection of the brain T1 + contrasting agent: the picture of the area of pathologic contrasting of the chiasmosellar region (red arrow) with elevated parameters of the perfusion. Probably, the picture of inflammatory (infectious?) alterations. Tumor presence is unlikely.
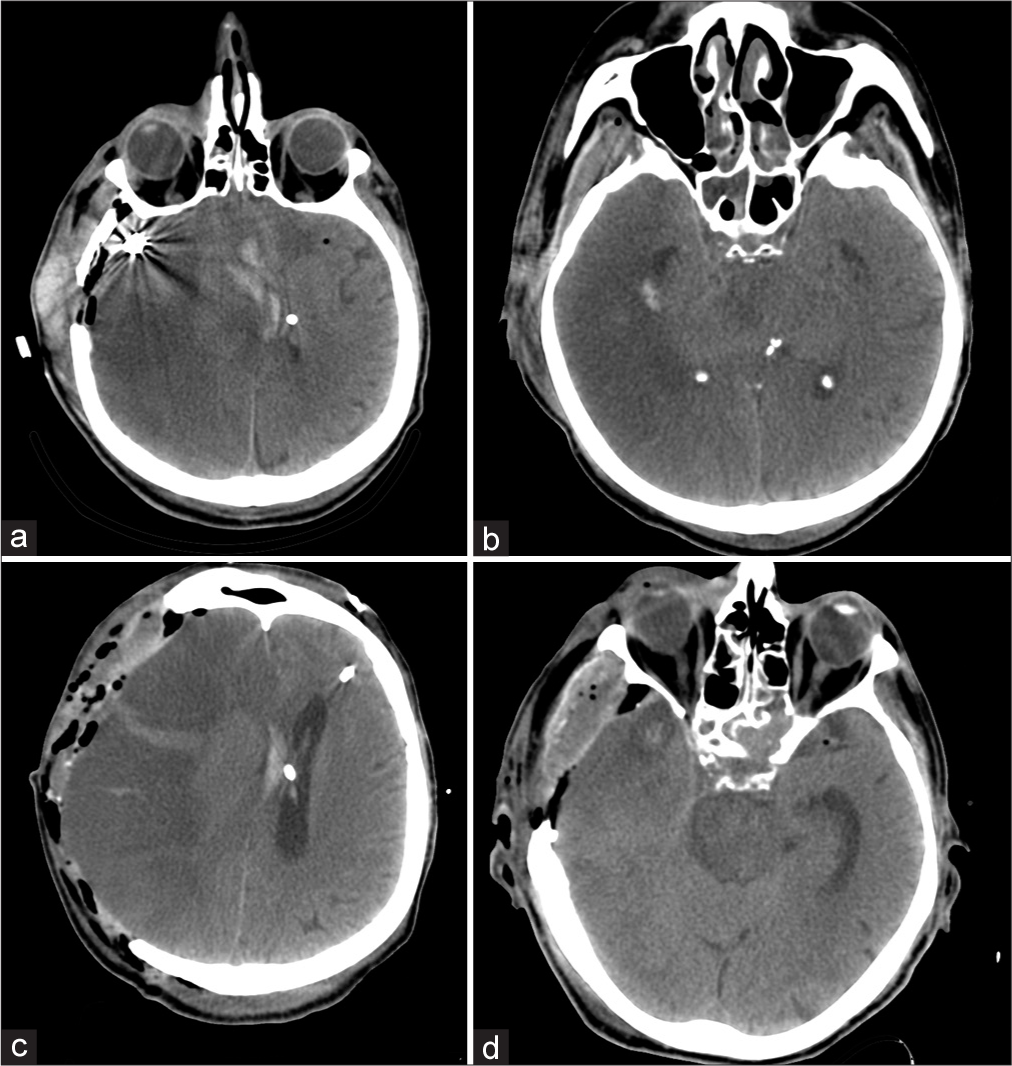
The patient’s condition worsened when he felt a sudden headache and left extremities weakness. The patient was urgently hospitalized at the Burdenko Neurosurgical Center, Moscow, Russia. Computed tomography angiography (CTA) of the head revealed subarachnoid-parenchymal hemorrhage (around 40 ml) with the formation of an intracerebral hematoma of the right frontotemporal lobe. Besides, multi-slice CTA showed a fusiform aneurysm of the M2 branch of the right MCA [
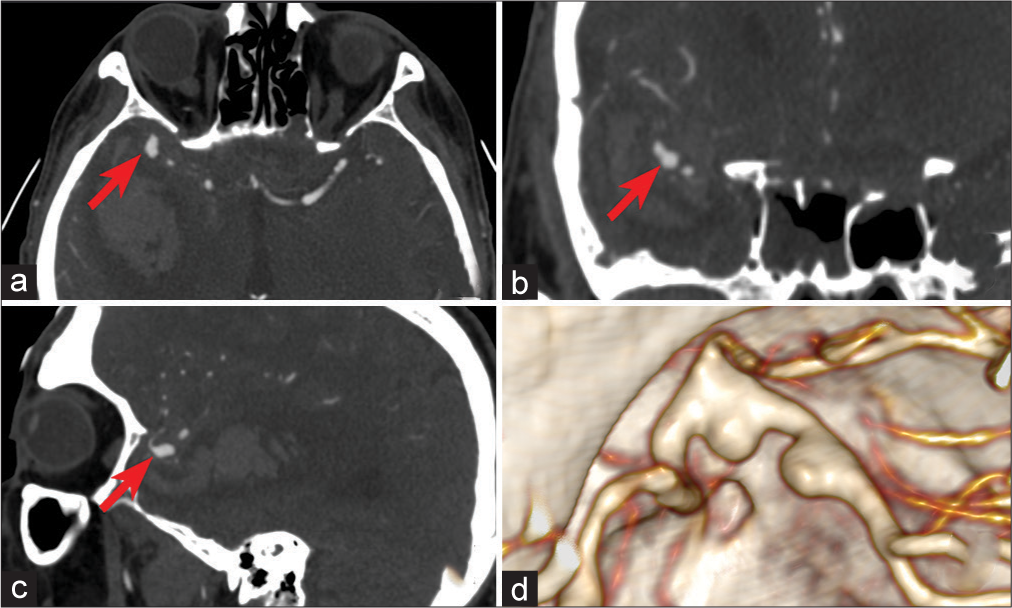
Figure 2:
(a-c) Axial, sagittal, and frontal computed tomography angiography projection of the head: Fusiform, partially thrombosed aneurysm of the right middle cerebral artery (MCA) (red arrow). A 3D reconstruction showed a contrasting part of the aneurysm in the area of M2 of the right MCA in the form of two bulgings with a wide neck.
During the comparison of the obtained MRI data and the association of the mass lesion with the sphenoid sinus, an infectious-inflammatory process was suspected. Nevertheless, considering the vital indications, an urgent microsurgical intervention for fusiform aneurysm of the right MCA was performed. Considering the localization and morphological peculiarities of the aneurysm, and acute period of hemorrhage, endovascular treatment was not recommended. The patient was prepared for microsurgical clipping of the aneurysm.
Microsurgical clipping of the fusiform-ectatic aneurysm of the right MCA in the conditions of rupture was performed. The manipulation was carried out under bispectral index neuromonitoring and ultrasonic dopplerography control of the blood flow. An extra-intracranial microanastomosis (EICMA) was formed on the right. An open biopsy of the neoplasm in the chiasmosellar region was made.
External ventricular drainage (EVD) was placed. Pterional trepanation with an infratemporal bone resection was performed from the arciform incision of the skin in the right frontotemporal area. The Dura mater was moderately tensed. It was opened with a semicircular incision to the forehead. In the area of the Sylvian fissure and the base, the signs of hemorrhage were visible. The brain was barely pulsing. In the pole of the temporal lobe on the right, 1.5 cm encephalotomy was performed. The hematoma cavity was opened. It contained soft clots with blood that were removed easily by suction and washing. Gradually, all available fragments of the hematoma (around 40 ml) were removed. The brain started to pulse. The surgeons opened the carotid cistern. Blood-containing liquor appeared. Stepwise, the Sylvian fissure was dissected for the isolation of the sclerosed internal carotid artery (ICA) and elongated and twisted MCA. The MCA was fusiform expanded in the area of the bifurcation. In the area of the bifurcation, a partially thrombosed aneurysm (around 1.5 cm in diameter) was revealed cuffed by an “old” hematoma with a capsule. The M1 segment was isolated. During the dissection of the aneurysm, arterial hemorrhage started. The rupture was revealed in the sidewall of the aneurysm. The hemorrhage was controlled by the suction and stopped by the application of the fragment of Tachocomb and gauze. Numerous scars and the sclerosed hematoma capsule indicated a hemorrhage occurred long ago. Major technical difficulties were faced in the isolation of the aneurysm that was a hemisphere-shaped ectasia on the sidewall of the twisted and expended MCA bifurcation. During the isolation of one of the branches of M2, another aneurysm rupture occurred associated with a temporary clipping of the M1 segment (2 × 3–4 min). The aneurysm was clipped with two standard clips Neuron. The ultrasonic dopplerography showed that one of the M3 branches functioned (20 cm/s), and the other coupled branch did not function despite the visually patent bifurcation lumen. Applications of papaverine, intra-arterial injection of prolapse, and reposition of the clips were not successful. The trapping of the M3 branch and arteriotomy (3 mm) were performed above the thrombosed M3 branch. It was revealed that the arterial wall was thickened and sclerosed. There was a newly formed clot in the lumen. The clots were removed, and the arteries were washed with an arterial microcatheter, which did not restore the blood flow. The artery was sutured, and the hemostasis was restored. EICMA was placed to compensate the blood flow. The surgeons isolated the frontal branch of the superficial temporal artery and the recipient artery, the distal segment of the thrombosed M3 branch. The microsurgical technique was used to make an endto-side anastomosis with Prolene 9-0. The checking of the anastomosis showed good blood flow (40 cm/s).
Further, the biopsy of resected aneurysm wall and soft tissue lesion in the chiasmosellar region were performed. The neoplasm was yellow and destroyed the bone plate of the skull base. A thin capsule of the neoplasm and several soft nonvascular tissue bioptates were taken. Biopsy results showed invasive fungal elements from outer to the inner side in the aneurysmal wall and numerous filamentary infiltrates with an acute angle branching confirming to the morphology of mycotic lesion (aspergillosis) [
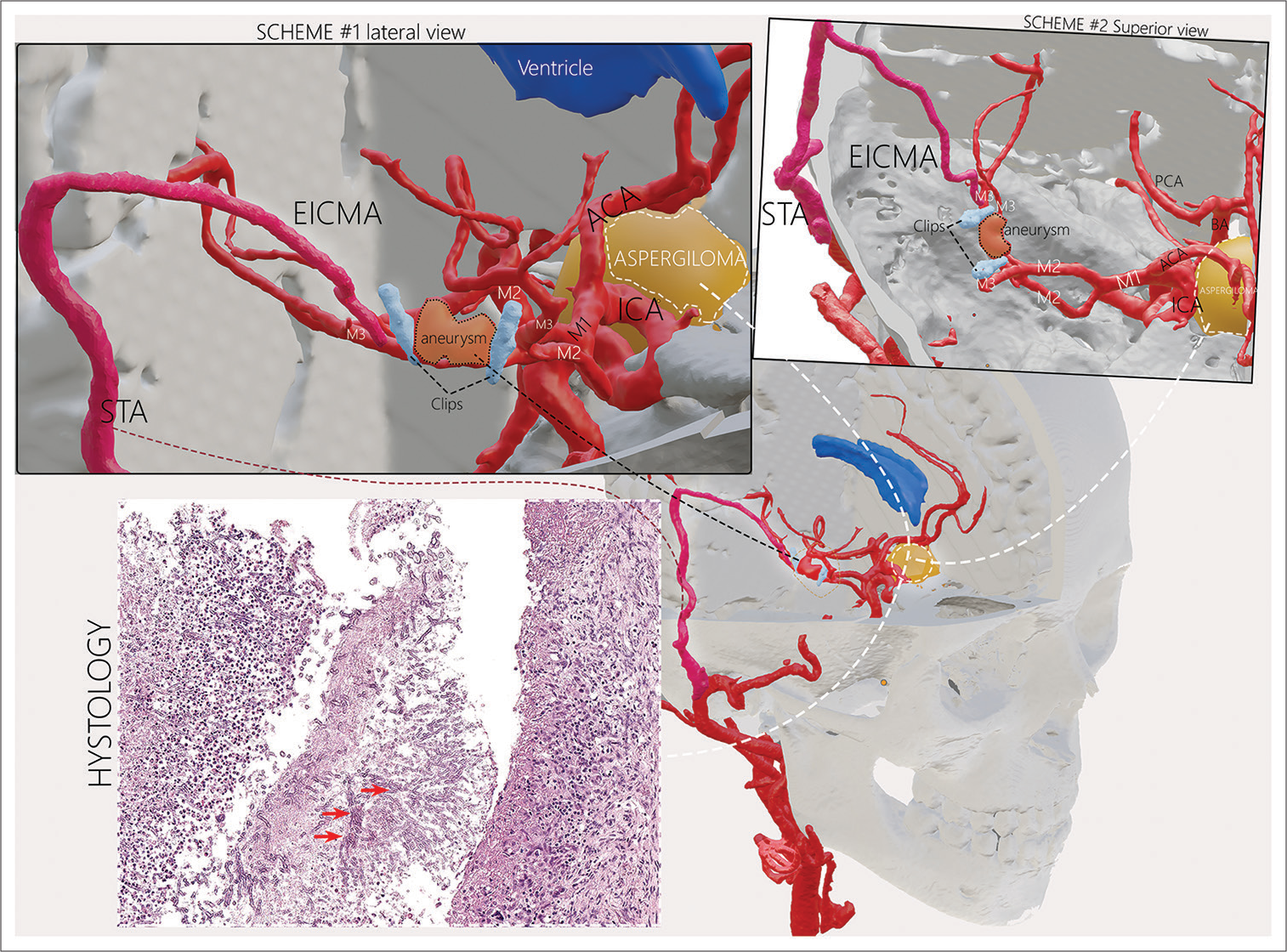
Figure 3:
The scheme of the surgical intervention: Trapping-clipping of the aneurysm, extra-intracranial microanastomosis of the superficial temporal artery, and the M3 branch of the right middle cerebral artery. The location of the aspergilloma in the chiasmosellar region. The microscopic study of the biological material revealed the fragments of the connective tissue with chronic granulomatous inflammation and branching hyphae of the fungus (red arrow).
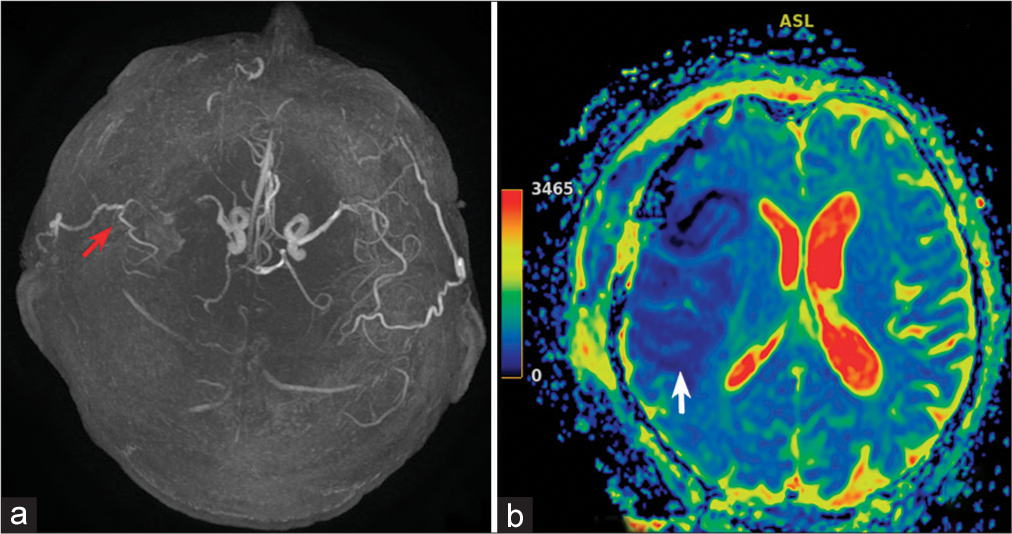
Despite satisfactory functioning EICMA, an ischemic stroke event developed in the basin of the right MCA artery after the clipping of the mycotic aneurysm. MRI and magnetic resonance arterial spin labeling perfusion on the 2nd day after the surgery [
A parenchymatous intracranial pressure (ICP) sensor was implanted. The ICP was monitored. On the 3rd day after the surgery, a persisting increase in the ICP of more than 30 mmHg was observed for an hour that was not resolved by conservative methods (EVD, osmotic diuretic, deep sedation).
Considering the dynamics of intracranial hypertension, an increase in the brain edema, and dislocation revealed by computed tomography (CT) [
Figure 5:
(a and b) computed tomography (CT) of the head on the 3rd day after the surgery. Hemispheric ischemic impairments on the right, expressed edema and dislocation of the brain. (c and d) CT of the head after decompressive trepanation. Expressed brain prolapse into the trepanation defect. Cisterna ambiens is visualized.
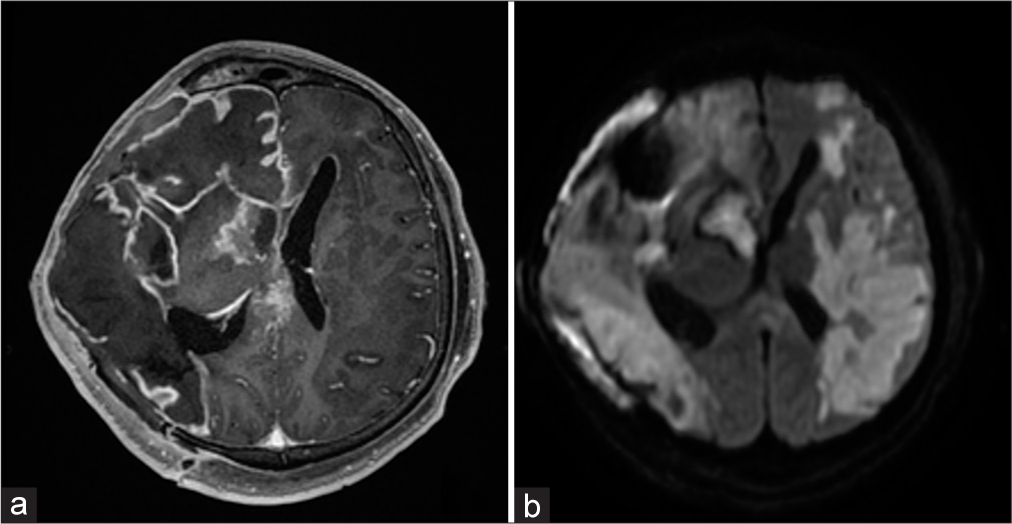
Despite the performed conservative therapy and relative stabilization of the patient, sub-febrile fever persisted for a month. Suddenly, exudation presented from under the skin flap. MRI of the brain [
Figure 6:
(a) Magnetic resonance imaging of the head with contrasting: Signs of expressed leptomeningitis with the formation of epidural empyema in the right frontal region. (b) Diffusion-weighted imaging: Vast foci of cerebrovascular accident are visualized in the basins of the middle cerebral artery from both sides. The internal carotid artery is occluded on the left.
After antibacterial and fungicide therapy, the inflammatory-exudative process resolved. Within a month, an observation and recreational therapy were performed that did not reveal the signs of infectious-inflammatory alterations. The patient was in the condition of low consciousness. He was transferred to the rehabilitation center without the signs of inflammation and underwent recreational therapy for several months without any expressed neurological dynamics. The patient died several months after the discharge from the rehabilitation center.
DISCUSSION
Invasive aspergillosis can be complicated by the formation of secondary intracranial mycotic aneurysms.[
Timely diagnostics of CNS aspergillosis is a complicated clinical task. It is confirmed by the fact that only in 56% of patients, CNS aspergillosis is diagnosed during life.[
CT and MRI signs of this disease are non-specific, which complicates the differentiation analysis of other mass lesions. CT scans show invasive aspergillosis as a well-isolated, heterogeneous, and hyperdense mass or heterogeneous infiltrate formation. Besides, there are signs of hyperostosis and osteolysis of the adjoining bone tissue. The presence of calcificates with a density of more than 2000 HU is a typical sign of aspergillosis.[
Often, the morphological study can provide false-negative results. Thus, it is recommended to take bioptates from several areas of the mass lesion. The involvement of a large amount of liquor and the performance of repeated microbiological studies can provide the possibility of the infectious agent identification.[
Alternative “non-culture-based” methods of verification of the infectious agent have higher sensitivity and specificity. They include galactomannan test that detects the antigens to the fungal cell walls and is widely used for the diagnostic of invasive aspergillosis and monitoring of the effectiveness of pharmacotherapy. The manufacturer recommends using the test for liquor. There are no respective standardized normative parameters. However, Antinori et al. demonstrated 87% sensitivity of this test in 15 patients with CNS aspergillosis.[
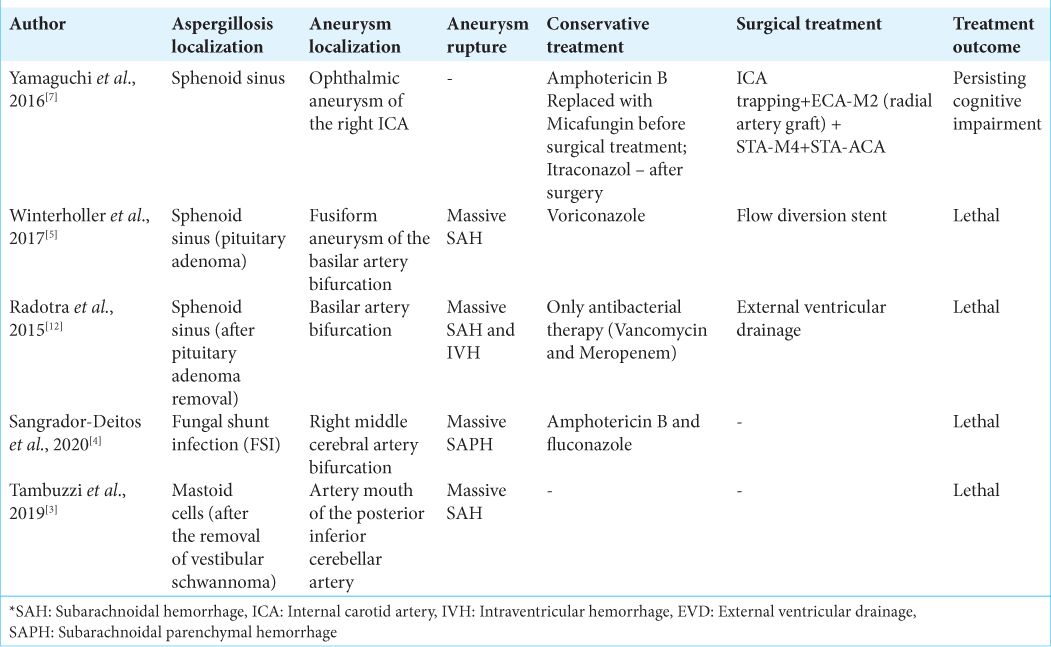
There are limited publications on this clinical issue. The majority of them are single clinical cases. We looked through literature for studies since 2015. They are summarized in [
Radotra et al.[
Yamaguchi et al.[
Winterholler et al.[
Rare localization of aspergillosis was described by Tambuzzi et al.[
Sangrador-Deitos et al.[
The experience of endovascular treatment in the acute period after the hemorrhage caused by CNS aspergillosis is scarce and required further studies. It is limited by single cases of deconstructive interventions in patients with mycotic pseudoaneurysm.[
CONCLUSION
Mycotic aneurysm caused by Aspergillus infection is a rare and dangerous complication associated with a high rate of lethality. A high risk of the lethal outcome in patients with invasive mycotic infection and rupture of mycotic aneurysm highlight the importance of timely diagnostics and the beginning of antimycotic therapy. The issue of the evaluation of the revascularization methods effectiveness in patients after surgical treatment of a mycotic aneurysm associated with cerebral aspergillosis remains poor.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Antinori S, Corbellino M, Meroni L, Resta F, Sollima S, Tonolini M. Aspergillus meningitis: A rare clinical manifestation of central nervous system aspergillosis. Case report and review of 92 cases. J Infect. 2013. 66: 218-38
2. Baeesa SS, Bakhaidar M, Ahamed NA, Madani TA. Invasive orbital apex aspergillosis with mycotic aneurysm formation and subarachnoid hemorrhage in immunocompetent patients. World Neurosurg. 2017. 102: 42-8
3. Bokhari R, Baeesa S, Al-Maghrabi J, Madani T. Isolated cerebral aspergillosis in immunocompetent patients. World Neurosurg. 2014. 82: e325-33
4. Hurst RW, Judkins A, Bolger W, Chu A, Loevner LA. Mycotic aneurysm and cerebral infarction resulting from fungal sinusitis: Imaging and pathologic correlation. Am J Neuroradiol. 2001. 22: 858-63
5. Jao SY, Weng HH, Wong HF, Wang WH, Tsai YH. Successful endovascular treatment of intractable epistaxis due to ruptured internal carotid artery pseudoaneurysm secondary to invasive fungal sinusitis. Head Neck. 2014. 36: 1391
6. Kami M, Ogawa S, Kanda Y, Tanaka Y, Machida U, Matsumura T. Early diagnosis of central nervous system aspergillosis using polymerase chain reaction, latex agglutination test, and enzyme-linked immunosorbent assay. Br J Haematol. 1999. 106: 536-7
7. Radotra BD, Salunke P, Parthan G, Dutta P, Vyas S, Mukherjee KK. True mycotic aneurysm in a patient with gonadotropinoma after trans-sphenoidal surgery. Surg Neurol Int. 2015. 6: 193
8. Sangrador-Deitos MV, Olvera JA, Espinal HA, Hernández GC, Morales VA, Hernandez JL. Fungal mycotic aneurysm in a patient with Aspergillus terreus chronic meningoencephalitis. Surg Neurol Int. 2020. 11: 1-4
9. Steiner I, Schmutzhard E, Sellner J, Chaudhuri A, Kennedy PG. EFNS-ENS guidelines for the use of PCR technology for the diagnosis of infections of the nervous system. Eur J Neurol. 2012. 19: 1278-91
10. Sulahian A, Porcher R, Bergeron A, Touratier S, Raffoux E, Menotti J. Use and limits of (1-3)-β-D-glucan assay (fungitell), compared to galactomannan determination (platelia Aspergillus), for diagnosis of invasive aspergillosis. J Clin Microbiol. 2014. 52: 2328-33
11. Tambuzzi S, Boracchi M, Maciocco F, Tonello C, Gentile G, Zoja R. Fungal aneurism of the right posterior inferior cerebellar artery (PICA). Med Mycol Case Rep. 2019. 26: 25-7
12. Winterholler M, Coras R, Geißdörfer W, Rammensee R, Gölitz P, Bogdan C. Fatal mycotic aneurysm of the basilar artery caused by Aspergillus fumigatus in a patient with pituitary adenoma and meningitis. Front Med. 2017. 4: 1-7
13. Yamaguchi J, Kawabata T, Motomura A, Hatano N, Seki Y. Fungal internal carotid artery aneurysm treated by trapping and high-flow bypass: A case report and literature review. Neurol Med Chir (Tokyo). 2016. 56: 89-94