- Discipline of Neurosurgery, Departament of Neurology, Campinas State University-SP, São Paulo, Brazil
- Discipline of Neurosurgery, Departament of Neurology, University of São Paulo-SP, São Paulo, Brazil
Correspondence Address:
Kleber P. Duarte
Discipline of Neurosurgery, Departament of Neurology, University of São Paulo-SP, São Paulo, Brazil
DOI:10.4103/2152-7806.188916
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Otávio T. da Silva, César C. de Almeida, Iglesio RF, de Navarro JM, Teixeira MJ, Duarte KP. Surgical variation of microvascular decompression for trigeminal neuralgia: A technical note and anatomical study. Surg Neurol Int 23-Aug-2016;7:
How to cite this URL: Otávio T. da Silva, César C. de Almeida, Iglesio RF, de Navarro JM, Teixeira MJ, Duarte KP. Surgical variation of microvascular decompression for trigeminal neuralgia: A technical note and anatomical study. Surg Neurol Int 23-Aug-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/surgical-variation-microvascular-decompression-trigeminal-neuralgia-technical-note-anatomical-study/
Abstract
Background:In this article, the authors described their experience in microvascular decompression for trigeminal neuralgia.
Methods:The microvascular decompression technique used in the authors’ institution is described in a step by step manner with some illustrative cases as well as a cadaver dissection to highlight the differences with other previously described techniques.
Results:Since 2013, 107 patients were operated in the Neurosurgery Division of the University of São Paulo using the described technique, with a shorter operative time and avoiding cerebellar retractor compared with classic techniques.
Conclusion:Our modified microvascular decompression technique for trigeminal neuralgia can be used with safety and efficiency for treating trigeminal neuralgia.
Keywords: Microvascular decompression, treatment, trigeminal neuralgia, surgery
BACKGROUND
Trigeminal neuralgia (TN) is a painful syndrome characterized by high intensity pain on the territory of trigeminal nerve, described as lightning bolt, paroxysmal, that can last for seconds to a few minutes, with well-defined triggers such as washing face or brushing teeth.[
The pioneer of surgery for trigeminal pain was Frazier[
In the classic surgical technique,[
There are innumerous variations of the technique, but none is perfect, with nuances and pitfalls in each technique. Hence, it depends on the expertise of a surgeon how to handle the treatment.[
This paper aims to present a modified variation of microvascular decompression, reaching the same objectives of classic technique.
TECHNICAL CONSIDERATIONS
Positioning
Before the anesthesia procedure, all patients undergo a neurologic exam performed in the surgical room and cervical amplitude evaluated to rotate in positioning. The operating table stays in the center of the room and the instrument table at the right side of the surgeon. Before any procedure, the patient receives a single dose of corticoid and antibiotic.
After general anesthesia and intubation, the patient's head is fixed with a framework 3-point head fixation. The sites of the pins are contralateral to the surgery site in order to avoid conflict with microscope; one in frontal and two occipital almost in midline. Legs are tapped to reduce the risk of deep vein thrombosis; no central vein catheter is used, and if temporal urinary catheter is placed, it is removed after the end of the anesthesia procedure.
The patient positioning is in the supine neutral position, with rotation of the head approximately 60 degrees away from the affected side. No rolls are used frequently, but some patients with cervical ankyloses may need one roll below scapula because of the risk of cervical injury. The patient is fixed with belts on the operating table to permit maximal rotation of the operating table during the surgery. No flexion/extension of the neck is necessary; the vertex head stays in the neutral position to avoid jugular compression.
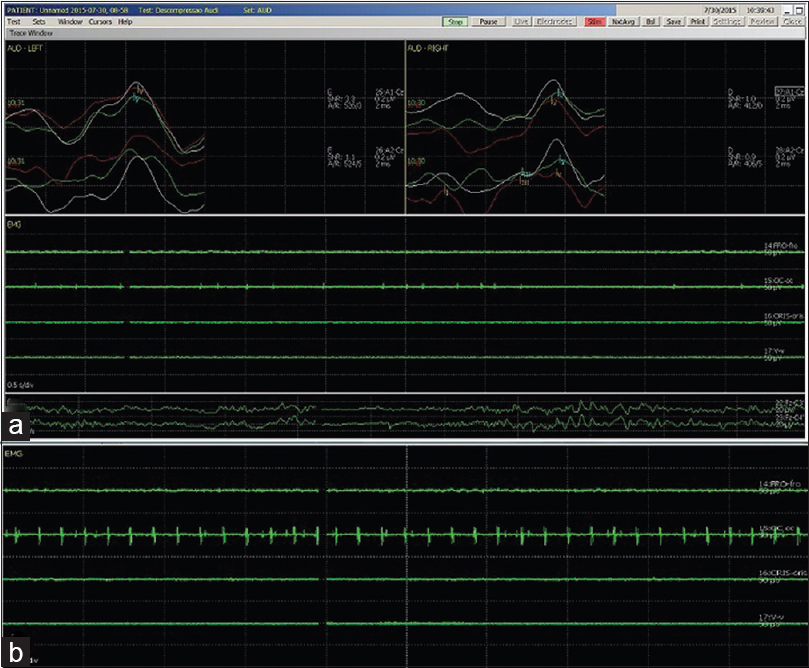
Trigeminal, facial, and acoustic nerves are monitored by an electrophysiologist to alert for any nerve damage; if some injury occurs, the surgery is stopped immediately and the surgical site is fully irrigated with a warm Ringer lactate solution until potential nerve recover [Figures
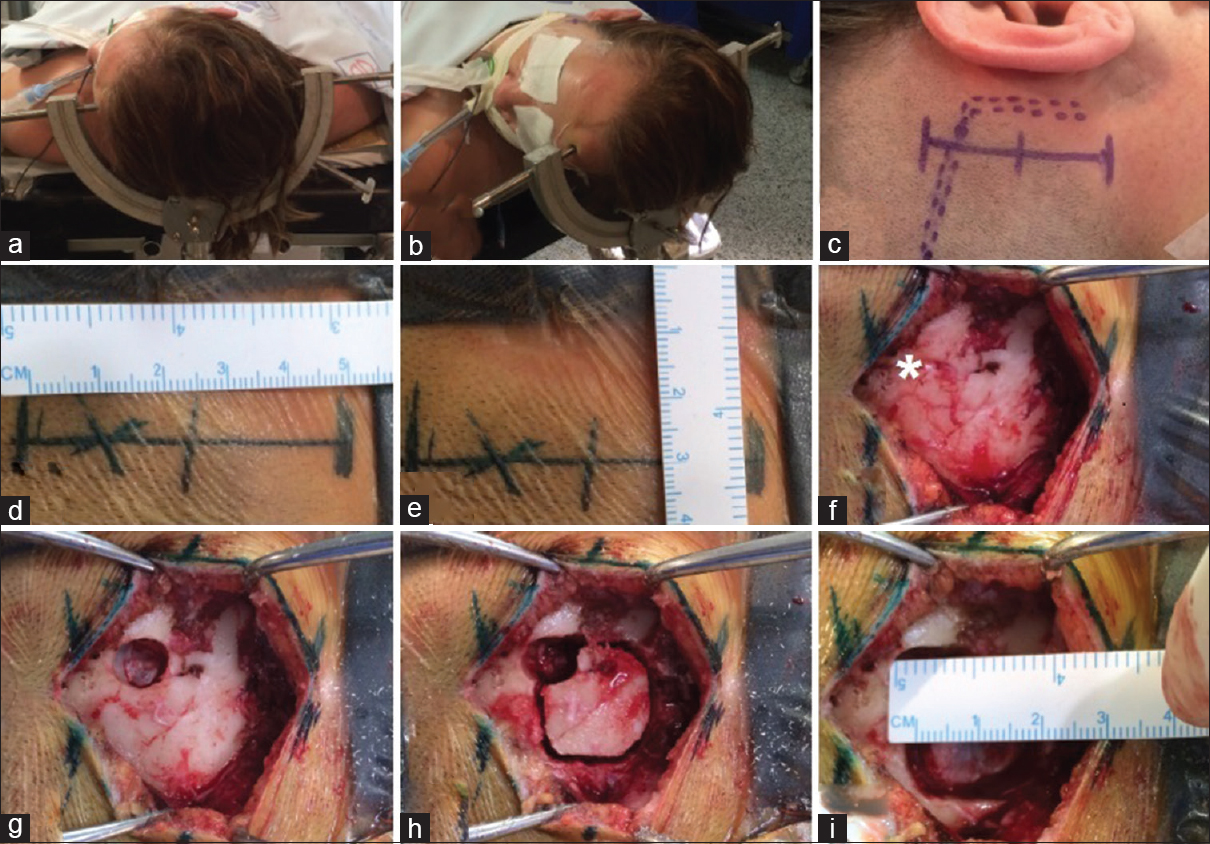
Figure 1
(a and b) The operating table stays at the center of the room and the patient is positioned in the supine position with 60° head rotation away from the surgery site. (c) Skin drawing showing anatomical landmarks. The straight full line corresponds to skin incision. The point line to corresponds to transverse sigmoid sinus. (d and e) A 4–5 cm length skin incision is marked and 3 cm retroauricular. (f) *corresponds to asterion, the junction of parietomastoideo, occiptomastoideo, and occiptoparietal sutures. (g) Single burr hole over the asterion and dura dissection before craniotomy to avoid inadverted durotomy. (h and i) A 2 cm craniotomy is performed in retromastoid, and single bone piece is removed. Bone corners are waxed to avoid cerebrospinal fluid leakage
Skin incision
Approximately 5 × 5 cm field of hair removal is performed behind the ear using a surgical clipper. Entire surgical field is cleaned with a solution of gluconate chlorexedine and afterward painted with alcoholic antiseptic solution.
The skin incision is marked with a sterile green dye. The junction of parietomastoideo, occiptomastoideo, and lambda suture is identified, referring to the asterion area, which shows the inferior corner of the transverse sigmoid junction.
The skin mark is a straight line drawn 3 cm behind the ear of the affected side of approximately 4–5 centimeters. The patient is covered with surgical fields and the surgical site with sterile drape; a skin incision with number 23 blade is performed. Subcutaneous and muscle dissections are made with monopolar electrocautery. One Gelpi retractor is used to keep the skin away.
Craniotomy and dural opening
A single burr hole is placed just below to the asterion in order to expose the transverse-sigmoid sinus junction. A 2 cm keyhole craniotomy is made, straight to the mastoid, with pneumatic high speed drill and bone stocked in fragments. The bone corner and mastoid cells are waxed to avoid bleeding during the microneurosurgery.
The inferior dura is anchored in bone with a polypropylene suture (Prolene®) 4-0 before opening. A single stitch with polypropylene is done at the center of the dura and pulled to lift the dura and any vessel in durotomy. A curvelineous durotomy is performed with an 11 blade, following the transverse sigmoid curvature. The dural edge is hit inferiorly and anchored with polypropylene suture.
Cisternal dissection
Gently, the cerebellar hemisphere is retracted with aspirator and bipolar, which is protected by cottonoids. Meticulous arachnoid dissection is made and CSF drained with aspiration. No coagulation is performed at this point. The objective is only to gain more space in the cisternal space of the CPA.
The cranial nerves is identified and the floculus indicates the VII/VIII nerves that emerges from the brainstem more posterior than trigeminal nerve. The arachnoid dissection of VII/VIII is performed to avoid retractions while mobilizing the arachnoid. The VII/VIII emerge from the bulbopontine sulci, medially to floculus, run into temporal bone that is fixed inside acoustic porus, and a light traction could lead to any damage, such as facial palsy or hypoacusia.
More superiorly and anterior, the trigeminal nerve is identified, in the cisternal portion, the V nerve merges latterly from pons at level of medium peduncle and run into the trigeminal porus, a dural fold that leads to Meckel cave. The superior petrosal vein or suprameatal tubercle could overlap the trigeminal nerve and obstruct the view of neurovascular complex, which when necessary, can be removed. The trigeminal nerve is dissected with a Penfield dissector, all arachnoid that surround the nerve is removed and most often neurovascular conflict is identified. Then the vessels are separated from the nerve and a Dacron vascular stent graft is placed over the REZ of the trigeminal nerve in contiguity to pons.
After the Dacron ring placement, all cisterns are filled with a Ringer lactate warmed solution. Prosthesis placement, hemostasis, and electrophysiologic monitoring are checked before the dural closure. If there is no vascular contact at REZ, arachnoid dissection and Dacron placement is performed anyway [Figures
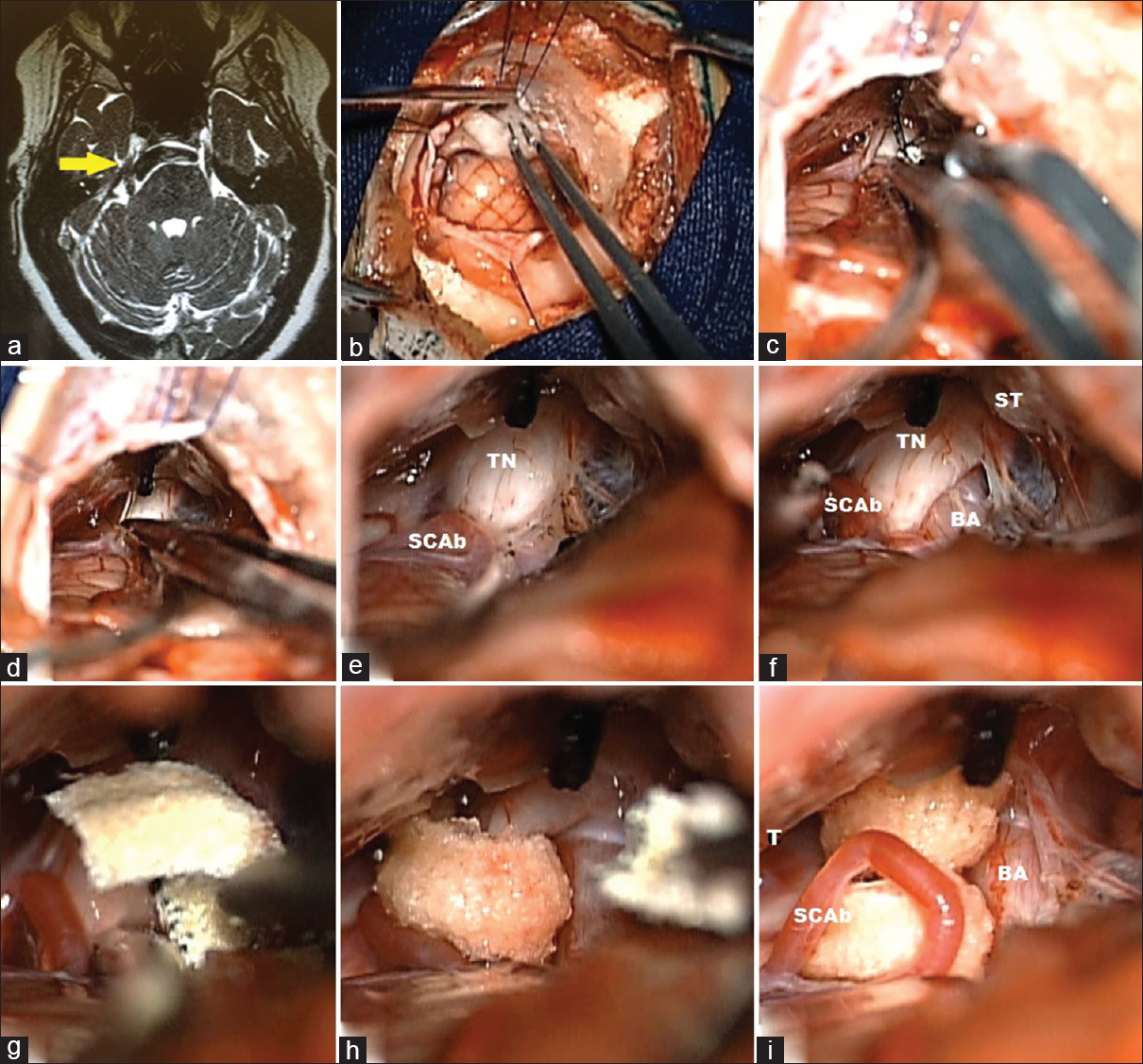
Figure 3
A female 52-year-old patient, with V3 right side trigeminal neuralgia, tried a balloon percutaneous rizotomy in 2013, in the epoch was treating a breast cancer. (a) MRI, T2 SPACE sequence, showing a right side basilar neurovascular conflict (yellow arrow). (b) Cisternal cerebrospinal fluid drainage after dural opening, cerebellar hemisphere is gently retracted with cottonoids and aspirator. (c and d) Identification of trigeminal nerve and coagulation of SPV to facilitate nerve dissection. (e) Identification of neurovascular conflict, basilar compressing the TN anteriorly and SCA branch touching posteriorly. (f) Dissection of terminal neuralgia in order to gain space to allocate Dacron. (h and i) Dacron placement separating vascular from neural structures
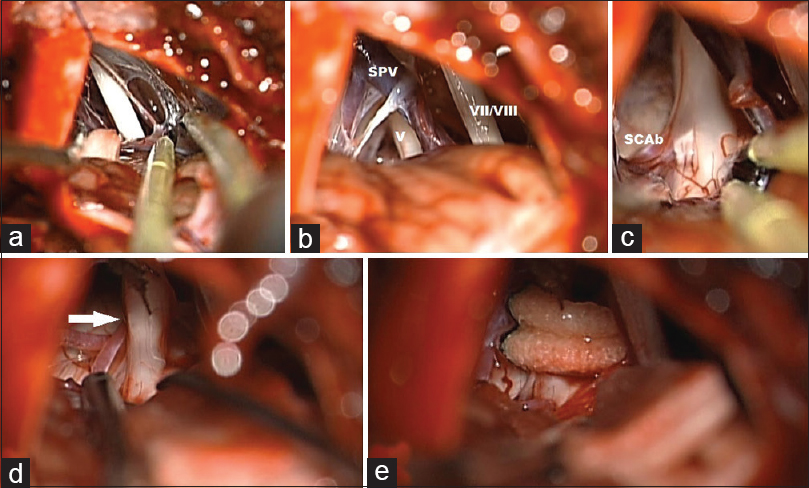
Figure 4
A 58-year-old male patient, with right side V3 trigeminal neuralgia (TN). (a) Dissection of VII/VIII complex to avoid arachnoid traction during the trigeminal nerve dissection. (b) After removal of arachnoid, the trigeminal nerve is identified showing neurovascular conflict. (c) TN compressed by a SCA branch. (d) White Arrow showing SCA branch separated from TN after dissection. (e) Dacron circular placement
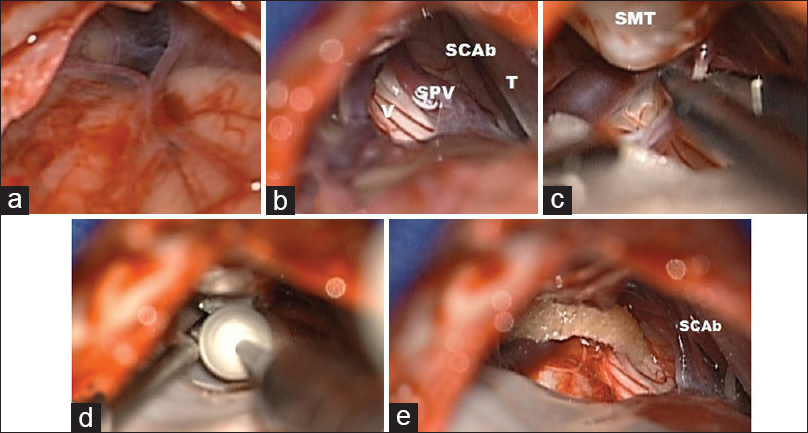
Figure 5
A 50-year-old female patient, with left side V2/V3 trigeminal neuralgia. (a) Cisternal view before APC cisternal dissection. Subarachnoid space showed without cerebellar retractors. (b) Cisternal view after CPA dissection, trigeminal nerve (V) shows a neurovascular complex with superior petrosal vein (SPV) posteriorly and superior cerebellar artery (SCAb) anteriorly, tentorium superiorly. (c) Coagulation of SPV. (d) Drilling suprameatal tubercule in order to gain more space to insert Dacron. (e) Dacron placement around trigeminal nerve. The SCA branch dissected from trigeminal nerve. Note that the circular format of Teflon reduces risk of detachment from nerve
Dural closure
Dura suture is made with polypropylene suture 4-0 in an uninterrupted fashion; the subdural space is filled with warmed physiological solution in order to remove air. The bone corner is waxed again to avoid CSF leakage or bleeding. A thick layer of absorbable hemostatic (Surgicell®) is placed over the dura and fibrin glue used to avoid small CSF leakages from needle passage. Small fragments of bone from craniotomy, that contain diploe and cortical layer, are placed in the craniotomy hole with fibrin glue to maintain bone position.
Skin closure
After skin retractors are removed, all hemostasis are reviewed, the muscle fascia is closed with nylon 3-0 in a separated manner to distribute muscle tension forces and maintain closure with neck movement. Subcutaneous is closed with simple polyglactin (Vicryl®) 3-0 points reversed in order to maintain proximity to the tissue and hide the knot through the wound.
We perform intradermal stitches with a polyglecaprone (Monocryl®) 3-0, in a continuous fashion, with good aesthetic results. The bandage is performed using sterile strip and micropore [
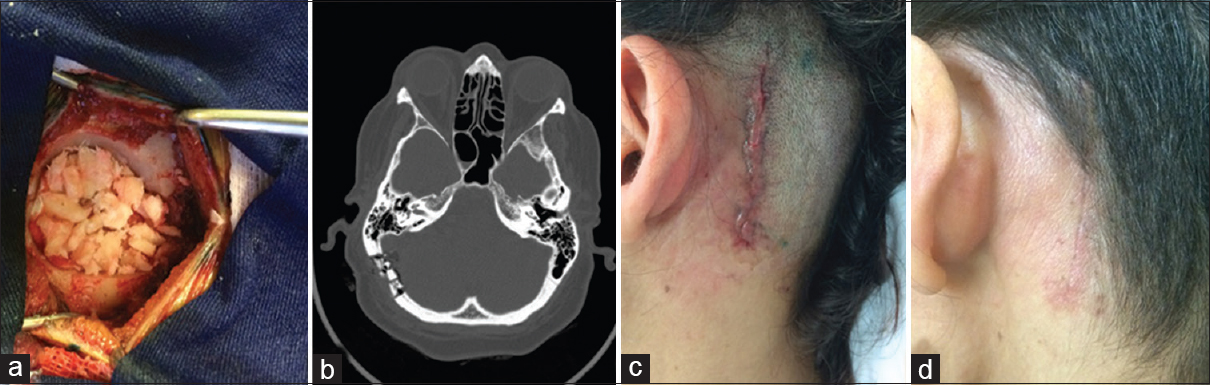
Figure 6
A case of 50-year-old female patient, with V2/V3 trigeminal neuralgia. (a) Bone fragments placement, small pieces with cortical/dipole covering the craniotomy. (b) CT image showing bone placement. (c) Intradermical stitches in first day postoperative. (d) Fourty-five days after surgery, with good aesthetical result
OBSERVATIONS
In our experience, with this new variation of the classic technique, we observed some good standpoints that must be related. The supine position, in addition to the lateral or prone position, promote less abdominal compression, less venous pressure, and consequently, less bleeding in surgery, facilitating CSF drainage in opening cisterns, needless CSF lumbar drain. Otherwise, is more easier to positionate in supine than prone or lateral, reducing the preoperatory time. Surgery time is aproximately 2–3 hours, and no central vein catheter is placed because the blood loss is minimal.
A small incision is preconized as a principle of minimal invasive surgery in order to reduce postoperative pain, avoid lesions of occipital nerve and artery, and to achieve better aesthetical result. Small craniotomy follows the same principle, not requiring more than 2 cm in the microscopic view to work in the upper floor of CPA, however, in neoplasic lesions such as neurinomas or meningeomas, small craniotomies could lead to some trouble in hemostasis and tumor resection.
The rotation of the head facilitates cerebellar fall and CSF drainage from the CPA; the use of retractor can promote cerebellar contusions and edema. In cadaveric study, the supine position facilitated the brainstem view to identify REZ. The lateral position facilitated the petrous incidence view and hid brainstem, requiring a retractor to view the REZ. The lateral position created wide rotating angle of the head; in some cases of acoustic neurinoma, it is important to open internal acoustic porus and remove intrameatal tumor [
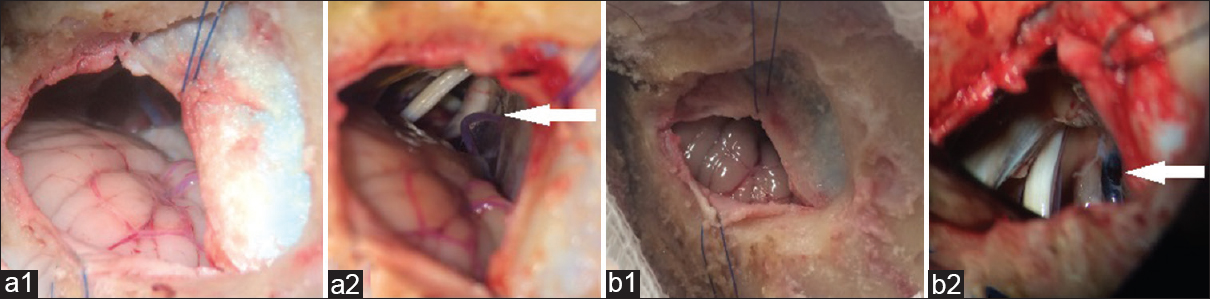
Figure 7
Cadaveric study. White Arrow indicates Trigeminal nerve and SPV. (a; 1, 2) Supine position. Cerebellar felt after opening dura and arachnoid dissection. The brainstem can be viewed in the macroscopic view; no brain retractor is needed, only gravitational force is acting over cerebellum. In microscopic view, the REZ is easily exposed to resolve the neurovascular conflict. (b; 1, 2) Lateral position. Cerebellum is obliterating the cerebellopontine angle view; in microscopic view, use of retractor are important to see trigeminal nerve origin, and the dura and SPV has more blood as a result of increase in intraabdominal pressure. In this position there is a better view of petrosal surface to work more distal parts of cranial nerves
Small fragments of bone are replaced with fibrin glue and have less bone reabsorption and homogenous solidification. The full bone can be replaced with plates, however, the bone gap can lead to reabsorption and can become prominent. Intradermal stitches preserve hair surrounding the incision and have excellent aesthetical result.
CONCLUSION
Since our service has changed the technical approach to MVD for TN, we achieved major advances in quality and surgical time. We started performing this technique in 2013 and have already treated more than 100 cases, operated with a total mean surgical time of 2.5 h. The patient positioning is much simpler than the classical technique, along with a smaller craniotomy, which facilitated even less experienced Neurosurgeons/Residents. Similar reports showed can reach same objectives with less complicated patient positioning and small craniotomies, such as that reported by Broggi et al. in his paper presenting a similar technique[
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Broggi G, Broggi M, Ferroli P, Franzini A. Surgical technique for trigeminal microvascular decompression. Acta Neurochir. 2012. 154: 1089-95
2. Cohen-Gadol AA. Microvascular decompression surgery for trigeminal neuralgia and hemifacial spasm: Naunces of the technique based on experiences with 100 patients and review of the literature. Clin Neurol Neurosurg. 2011. 113: 844-53
3. Cruccu G, Gronseth G, Alksne J, Argoff C, Brainin M, Burchiel K. AAN-EFNS guidelines on trigeminal neuralgia management. Eur J Neurol. 2008. 15: 1013-28
4. Dandy WE. The treatment of trigeminal neuralgia by the cerebellar route. Ann Surg. 1932. 96: 787-95
5. Forbes J, Cooper C, Jermakowicz W, Neimat J, Konrad P. Microvascular decompression: Salient surgical principles and technical nuances. J Vis Exp. 2011. p. e2590-
6. Frazier CH. Operation for the Radical Cure of Trigeminal Neuralgia: Analysis of Five Hundred Cases. Ann Surg. 1928. 88: 534-47
7. Gronseth G, Cruccu G, Alksne J, Argoff C, Brainin M, Burchiel K. Practice parameter: The diagnostic evaluation and treatment of trigeminal neuralgia (an evidence-based review): Report of the Quality Standards Subcommittee of the American Academy of Neurology and the European Federation of Neurological Societies. Neurology. 2008. 71: 1183-90
8. Ibrahim S. Trigeminal neuralgia: Diagnostic criteria, clinical aspects and treatment outcomes. A retrospective study. Gerodontology. 2014. 31: 89-94
9. Jannetta PJ. Observations on the etiology of trigeminal neuralgia, hemifacial spasm, acoustic nerve dysfunction and glossopharyngeal neuralgia. Definitive microsurgical treatment and results in 117 patients. Neurochirurgia. 1977. 20: 145-54
10. Jannetta PJ. Treatment of trigeminal neuralgia by suboccipital and transtentorial cranial operations. Clin Neurosurg. 1977. 24: 538-49
11. Jannetta PJ, McLaughlin MR, Casey KF. Technique of microvascular decompression. Technical note. Neurosurg Focus. 2005. 18: E5-
12. Ma Z, Li M, Cao Y, Chen X. Keyhole microsurgery for trigeminal neuralgia, hemifacial spasm and glossopharyngeal neuralgia. Eur Arch Otorhinolaryngol. 2010. 267: 449-54
13. Maarbjerg S, Wolfram F, Gozalov A, Olesen J, Bendtsen L. Significance of neurovascular contact in classical trigeminal neuralgia. Brain. 2015. 138: 311-9
14. McLaughlin MR, Jannetta PJ, Clyde BL, Subach BR, Comey CH, Resnick DK. Microvascular decompression of cranial nerves: Lessons learned after 4400 operations. J Neurosurg. 1999. 90: 1-8
15. Obermann M. Treatment options in trigeminal neuralgia. Ther Adv Neurol Disord. 2010. 3: 107-15
16. Reddy GD, Viswanathan A. Trigeminal and glossopharyngeal neuralgia. Neurol Clin. 2014. 32: 539-52
17. Sade B, Lee JH. Microvascular decompression for trigeminal neuralgia. Neurosurg Clin N Am. 2014. 25: 743-9
18. van Kleef M, van Genderen WE, Narouze S, Nurmikko TJ, van Zundert J, Geurts JW. 1. Trigeminal neuralgia. Pain Pract. 2009. 9: 252-9
19. Vitali AM, Sayer FT, Honey CR. Recurrent trigeminal neuralgia secondary to Teflon felt. Acta Neurochir. 2007. 149: 719-22