- Department of Neurosurgery, Tokuda Hospital, Sofia, Bulgaria
- Department of Neurosurgery, Military Medical Academy, Sofia, Bulgaria
Correspondence Address:
Toma Y. Spiriev
Department of Neurosurgery, Military Medical Academy, Sofia, Bulgaria
DOI:10.4103/2152-7806.199555
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Vladimir S. Nakov, Toma Y. Spiriev, Ivan T. Todorov, Plamen Simeonov. Technical nuances of subtemporal approach for the treatment of basilar tip aneurysm. 06-Feb-2017;8:15
How to cite this URL: Vladimir S. Nakov, Toma Y. Spiriev, Ivan T. Todorov, Plamen Simeonov. Technical nuances of subtemporal approach for the treatment of basilar tip aneurysm. 06-Feb-2017;8:15. Available from: http://surgicalneurologyint.com/surgicalint_articles/technical-nuances-of-subtemporal-approach-for-the-treatment-of-basilar-tip-aneurysm/
Abstract
Background:Basilar tip aneurysms are one of the most complex vascular lesions to treat surgically because of their location, depth of the approach, and close proximity of vital neurovascular structures such as the mesencephalon, cranial nerves, perforating arteries to the thalamus. There are different surgical approaches utilized to reach basilar tip aneurysms, namely, pterional, pretemporal, orbitozygomatic, subtemporal, and anterior petrosectomy. Each of them has its advantages and limitations.
Methods:In this paper, we present our personal experience with the use of subtemporal approach. The technique is described in detail including its nuances and potential pitfalls.
Results:The subtemporal approach is indicated for basilar tip aneurysms located at the level of the floor of the sella turcica to 1 cm above the dorsum sellae.
Conclusion:Subtemporal approach offers good surgical corridor for the management of these complex vascular lesions.
Keywords: Aneurysmal clipping, basilar artery aneurysms, Osirix software, subtemporal approach
INTRODUCTION
Posterior circulation aneurysms account for approximately 15% of all intracranial aneurysms, however, when ruptured are related to higher morbidity and mortality due to their location deep within the brain and close proximity to vital neurovascular structure.[
There are various surgical approaches described for the treatment of basilar tip aneurysms, such as pterional, pretemporal, orbitozygomatic, transcavernous, subtemporal, anterior and petrosectomy.[
RELEVANT SURGICAL ANATOMY
The floor of the middle cranial fossa is formed by the tentorium, the superior surface of the petrous bone, and the intracranial surface of the greater sphenoid wing. In axial plane, the middle fossa floor is on the same level as the superior surface of the root of the zygomatic arch. The interpeduncullar cistern is projected in coronal plane on the temporomandibular joint because the anterior border of the cistern corresponds to the posterior third of proc. mandibulars of the zygomatic arch.[
The main structure forming the interpeduncular cistern is the Lilliequist membrane.[
The high of the basilar artery bifurcation is individual, however, in 87% of the adult population, it is located 1 cm below or at the level of dorsum sellae [
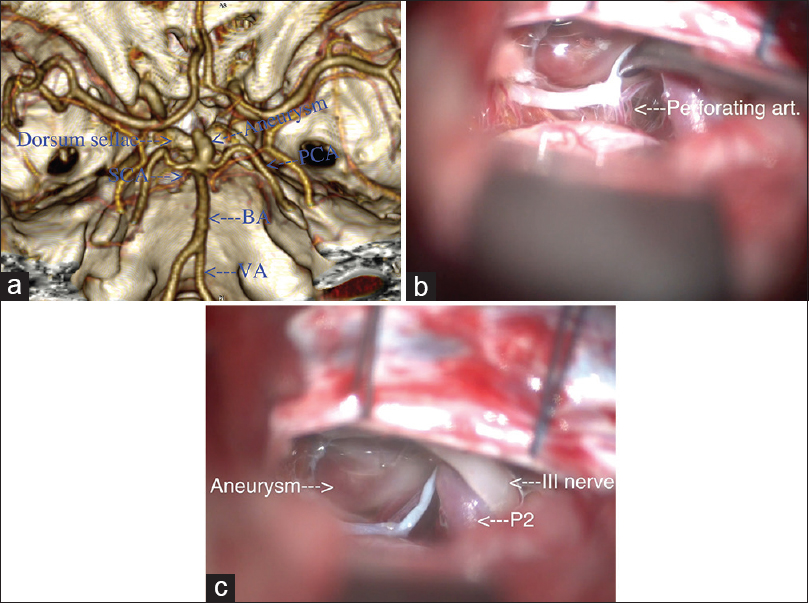
Figure 1
(a) Three-dimensional reconstruction using OsiriX software[
The trochlear and the oculomotor nerves has parallel trajectory. The trochlear nerve is located dorsal and lateral compared to the oculomotor nerve. The oculomotor nerve pierces the dura mater of the cavernous sinus in the area of the oculomotor triangle [
PREOPERATIVE CONSIDERATION
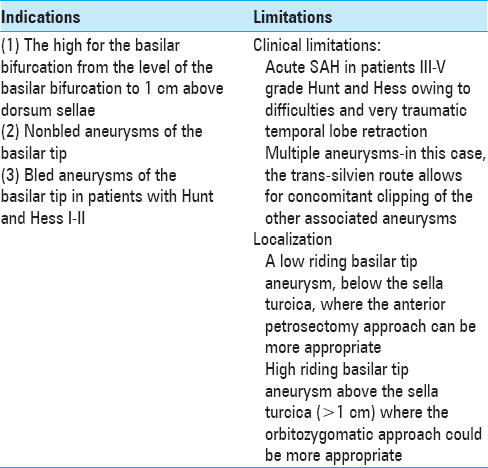
A detailed preoperative radiological evaluation is planned including computed tomography angiography (CTA), digital subtraction angiography (DSA) (in all ruptured basilar tip aneurysms), and magnetic resonance imaging (MRI) (in nonruptured aneurysms). The aneurysm anatomy is studied in relation to its position, dome configuration and location, neck size (length of the aneurysm clip planning), and anatomy of the ipsi/contralateral PcomA (use of fenestrated clip). The location of the aneurysm in relation to the posterior clinoid process is very important – very high riding aneurysm might not be suitable for subtemporal approach and may require pterional, OZ or pretemporal approach; on the contrary, very low riding aneurysms might require anterior petrosectomy technique in order to reach the lesions [
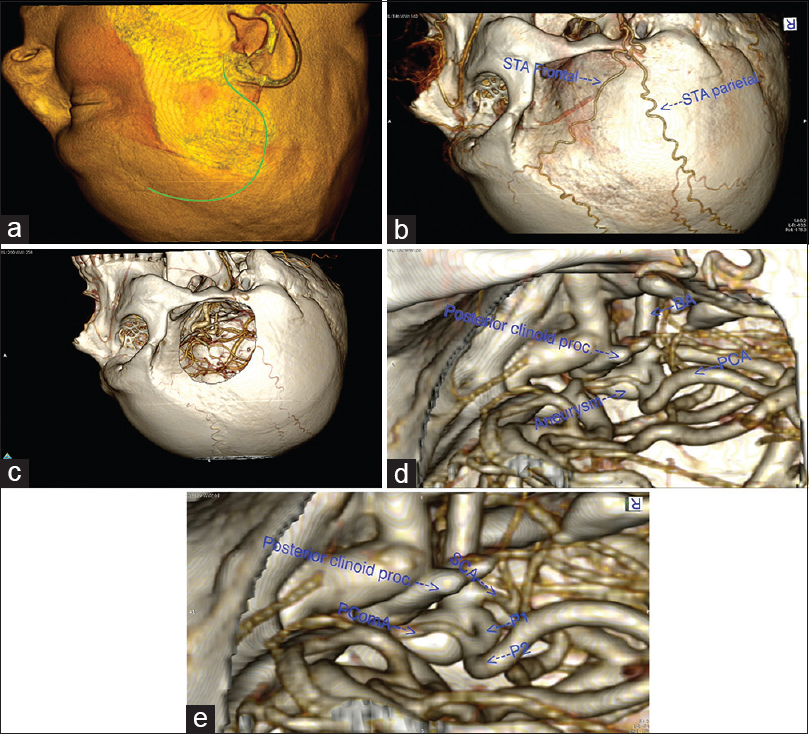
The Digital Imaging and Communications in Medicine (DICOM) files of 1 mm thickness and non-overlapping CTA slices are imported into OsiriX open-source imaging software (Version 5.8.1; free download from
Figure 2
(a) Preoperative simulation of the patient's head positioning and skin incision using OsiriX software.[
Using OsiriX software and its functions in three-dimensional (3D) volumetric rendering, one can easily recreate the craniotomy in the 3D work screen. There are two main possible functions:
utilizing the “Crop” tool with the cropping cube; by reducing the walls of the cube one can recreate a bone window to the aneurysm Using the “Clipping mode” where one could represent only the desired corridor to the lesion, and have a much better understanding of the lesion itself by selectively eliminating the surrounding tissues.
These two techniques are very fast and can provide a lot of useful information regarding the anatomy of the aneurysm. Detailed description of the technique of 3D reconstruction in OsiriX is not the subject of this paper and can be found elsewhere.[
DESCRIPTION OF THE SURGICAL TECHNIQUE [VIDEO 2]
We use the lateral position with the superior part of the operating table tilted as the patient's head is above the level of the heart.[
CRANIOTOMY
The surgical incision is of a “question mark” type, followed by interfascial temporalis dissection,[
The craniotomy is 5/5 cm in dimensions, located below the superior temporal line and centered in the coronal plane above the root of the zygoma [
ARACHNOID DISSECTION
We incise the dura C-shape fashion centered toward the cranial base. The intradural part of the operation is done under microscopic magnification. After evacuation of approximately 40 ml of CSF from the spinal drainage, the temporal lobe is gradually retracted. We use wide 1.2 cm brain spatula placed on the level of the root of the zygoma. The Labbé vein is located dorsally and is at a sufficient distance from the working field in order not to be retracted and exposed to injury. Other small venous tributaries from the temporal lobe to the middle cranial fossa that interferes with the surgical corridor to the aneurysm could be coagulated and sectioned. Using scalpel No. 11 the arachnoid of the ambient cistern is incised. This allows for more release of CSF and additionally reduces the temporal lobe retraction. After opening of the ambient cistern, the P2, SCA, and between them the oculomotor nerve can be identified. We widely open the arachnoid above the nerve in order to visualize the PcomA and its perforating arteries, which point superior and posteriorly. Medial to the third cranial nerve a thickened arachnoid is usually found composed of two layers, which is actually the lateral wall of the interpeduncullar cistern. After incision of this arachnoid, the aneurysmal fundus is visualized. At this moment, we cease the dissection in this direction and point our attention towards the space below and posterior to the third cranial nerve to provide access to the basilar artery (BA) and the aneurysm. Most often during the dissection below the nerve, the space is limited from the tentorial edge [
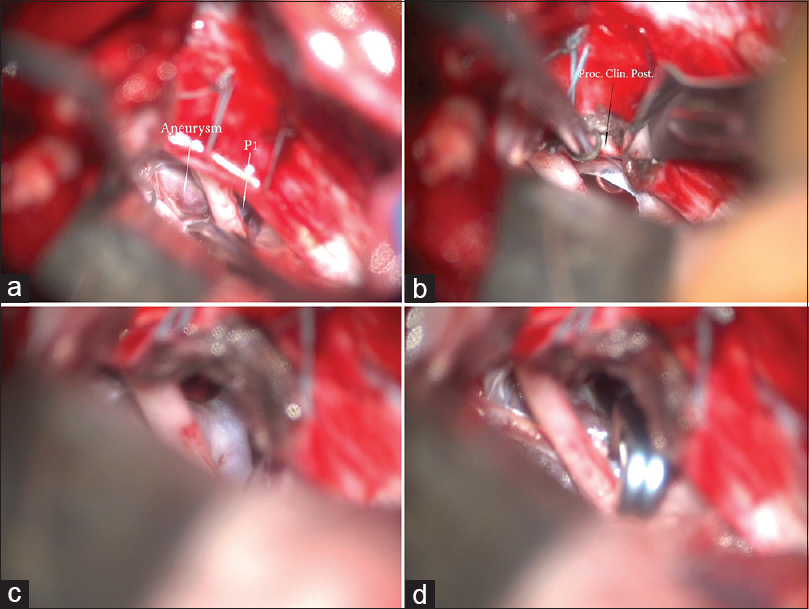
Figure 3
(a) Surgical view through right subtemporal approach. Two sutures are placed on the tentorium. The basilar tip aneurysm is visualized as well as the P1. Between them the oculomotor nerve courses and pierces the dura in the oculomotor triangle. (b) Splitting of the tentorium between the two sutures. This maneuver increases the visualization and exposes the posterior clinoid process. (c) After the tentorium is split, the neck of the aneurysm is exposed, as well as P1. (d) Surgical view after successful clip placement
Following the SCA in the medial direction and the incision of the abovementioned thickened arachnoid, defining the lateral edge of the interpeduncular cistern below the third cranial nerve, the BA is reached, as well as the SCA, P1, and the aneurysmal fundus. In this manner, two working channels are formed – below and above the III cranial nerve. Above the oculomotor nerve, a space is formed which is limited by the uncus of the parahypocampal gurys, posterior clinoid process, and the mammillary bodies. In the superior space PcomA, P2 and the perforating arteries are located. In the inferior working space, the SCA and the BA are found. The location of the aneurysmal neck and the beginning of the P1 depends on the high bifurcation of the BA and the bifurcation angle of both P1 arteries. Depending on the direction of the aneurysm, the aneurysmal fundus can be: (1) Adherent to the sella turcica (in case of aneurysm pointing anteriorly), (2) mammillary bodies and hypothalamus (aneurysm pointing superiorly), and (3) mesencephalon (aneurysm pointing posteriorly).
ANEURYSMAL DISSECTION
The aneurysm dissection starts from its neck. Adhesions are rarely located around the neck, independent of the direction of the aneurysmal sac. According to our experience, there is always space between the aneurysmal neck and the mesencephalon posteriorly, as well as the aneurysmal neck and dorsum sellae anteriorly.
In order to dissect the perforating vessels located behind the aneurysmal neck, we develop the arachnoid plane above ipsilateral P1 using microscissors, microdissector, and ball angle microdissector. Exposing the anterior and inferior surface of P1, an arachnoid plane is created, which is continued towards the BA and the aneurysmal fundus. Following this plane, it is easy to dissect the aneurysm neck from the arachnoid in the following order: (1) Ipsilateral – from the ipsilateral P1; (2) anterior – from the dorsum sellae with attention to dissect the aneurysm neck only, without the fundus, as the later can be adherent to the dorsum sellae; (3) posterior –the arachnoid together with perforating vessels as curtain is detached from the aneurysmal neck as the arachnoid serves as a protective layer for perforating arteries; (4) contralateral – with the angle ball-tip dissector anterior and posterior corridors are developed in contralateral direction around the aneurysm neck, as this manoeuver should be done very carefully to avoid premature aneurysm rupture. During the dissection of the contralateral site of the aneurysm, an attempt can be made to palpate the contralateral P1 to find free space between the aneurysm neck and the contralateral P1.
Keeping the dissection close to the wall of the aneurysm neck allows mobilizing the contralateral arachnoid together with the contralateral P1 perforating arteries away from the aneurysm neck. If the aneurysm is pointed posteriorly, the dissection behind the aneurysmal neck requires careful retraction of the cerebral peduncle because the aneurysm is embedded in the interpeduncullar fossa and there is no visibility. When the bifurcation angle of the BA is sharp, it is not always possible to perform the above described dissection due to adhesions of the ipsilateral and/or contralateral P1 towards the neck and the fundus of the aneurysm. In such cases, we dissect the space in front of P1 and the aneurysmal neck, and after that behind the ipsilateral P1 and behind the perforating arteries of P1. This dissection space is created behind the aneurysmal neck and the perforating arteries of P1, and in front of the perforating arteries of the BA in order to place fenestrated clip. The aneurysm clip window envelops the ipsilateral P1 together with its perforating vessels and the perforating vessels of the BA are left behind the clip towards the mesencephalon. It is important to dissect the perforating vessels behind the aneurysm at a sufficient distance of their length in order not to be bended or kinked by the aneurysm clip.
CLIPPING OF THE ANEURYSM
When the dissection of the aneurysm neck is completed from every site, a pilot clip could be placed. The aneurysms of the basilar tip are characterized by a wider neck in the coronal plane than in the sagittal plane.[
IN CASE OF INTRAOPERATIVE RUPTURE, WE DO THE FOLLOWING STEPS [VIDEO 3]
(1) We place the clip above the aneurysm neck, independent of the rupture site; (2) If this manoeuver is unsuccessful, we place a temporary clip on the BA, as the place of the temporary clip position is between the BA and SCA or proximal to the SCA; (3) If the bleeding continues, we make an attempt to dissect the aneurysm in the area of the rupture to visualize the opening of the rupture from every possible angle. We control the bleeding using the aspiration cannula and place a miniclip on the rupture site; (4) After cease of the bleeding, we dissect the aneurysm neck and place a clip over it and removing all the temporary clips. After we have assured successful clipping, we wait for controlled increase of the systolic pressure to at least 20 Torr above the normal systolic pressure of the patient.
COMPLICATION AVOIDANCE
The preoperative angiographic evaluation of the position of both P1 – the Towne view is very useful. Lateral position for better venous outflow and control of the spinal drain. Sharp and wide dissection above and below the third cranial nerve in order to create two wide working fields in case of intraoperative aneurysm rupture. Following of the arachnoid plane consequently towards the wall of ipsilateral P1, the wall of BA, aneurysm neck, and fundus. Dissection of the perforating vessels behind the aneurysm on sufficient distance of their length in order to avoid their bending or kinking. Dissection and clipping of the aneurysm neck without temporary occlusion of the BA. We utilize temporary occlusion only in cases of impossible dissection despite the use of all the abovementioned techniques. Clipping through the space above or below the third cranial nerve depending on the height of the bifurcation of the BA and the bifurcation angle of the artery.
COMPARISON WITH THE PTERIONAL APPROACH
According to generally accepted principles the aims of the approach in the aneurysmal surgery are:
The approach must have the shortest possible trajectory towards the target The approach must take a short amount of time The approach must be extra-axial, respecting the integrity of the brain The approach must allow access to afferent arteries and to the aneurysmal neck, after that to the efferent arteries, and after that to the aneurysm fundus.
When the aneurysm points upwards and posteriorly, the subtemporal approach has all the prerequisites in comparison to the trans-silvien approach. In the aneurysms that point anteriorly toward the basilar tip, one of the major disadvantages of the subtemporal approach seen is the lack of visibility of the contralateral P1. In this type of aneurysms, the contralateral P1 is visualized after the pilot aneurysm clip is placed. With the use of the trans-silvien approach in aneurysms pointing anteriorly, this disadvantage is overcome and it provides visibility of the contralateral P1. However due to its trajectory – pointing anteriorly and superiorly – first the aneurysm fundus is visualized and after that the aneurysm neck.
CONCLUSION
Subtemporal approach for the treatment of basilar tip aneurysms offers a direct route for this vascular lesion. A detailed understanding of the aneurysm, in particular its anatomy in relation to the bone and associated vessels anatomy, as well as careful evaluation of the preoperative radiological studies and meticulous following of microsurgical steps in the aneurysmal preparation and clipping is of great importance for the correct planning of the approach and avoiding intraoperative complications.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Videos Available on: www.surgicalneurologyint.com
References
1. Aziz KM, van Loveren HR, Tew JM, Chicoine MR. The Kawase approach to retrosellar and upper clival basilar aneurysms. Neurosurgery. 1999. 44: 1225-34
2. Barrow DL, Spetzler RF. Cotton-clipping technique to repair intraoperative aneurysm neck tear: A technical note. Neurosurgery. 2011. 68: 294-9
3. Dias DA, Castro FLO, Yared JH, Lucas Júnior A, Ferreira Filho LA, Ferreira NFPD. Liliequist membrane: Radiological evaluation, clinical and therapeutic implications. Radiol Brasil. 2014. 47: 182-5
4. Dolenc VV, Skrap M, Sustersic J, Skrbec M, Morina A. A transcavernous-transsellar approach to the basilar tip aneurysms. Br J Neurosurg. 1987. 1: 251-9
5. Figueiredo EG, Tavares WM, Rhoton AL, de Oliveira E. Nuances and technique of the pretemporal transcavernous approach to treat low-lying basilar artery aneurysms. Neurosurg Rev. 2010. 33: 129-35
6. Figueiredo EG, Zabramski JM, Deshmukh P, Crawford NR, Spetzler RF, Preul MC. Comparative analysis of anterior petrosectomy and transcavernous approaches to retrosellar and upper clival basilar artery aneurysms. Neurosurgery. 2006. 58: ONS13-21
7. Froelich SC, Abdel Aziz KM, Cohen PD, van Loveren HR, Keller JT. Microsurgical and endoscopic anatomy of Liliequist's membrane: A complex and variable structure of the basal cisterns. Neurosurgery. 2008. 63: ONS1-8
8. Hernesniemi J, Ishii K, Karatas A, Kivipelto L, Niemela M, Nagy L. Surgical technique to retract the tentorial edge during subtemporal approach: Technical note. Neurosurgery. 2005. 57: E408-
9. Hernesniemi J, Ishii K, Niemela M, Kivipelto L, Fujiki M, Shen H. Subtemporal approach to basilar bifurcation aneurysms: Advanced technique and clinical experience. Acta Neurochir Suppl. 2005. 94: 31-8
10. Hernesniemi J, Korja M. At the apex of cerebrovascular surgery--basilar tip aneurysms. World Neurosurg. 2014. 82: 37-9
11. Morita A, Kirino T, Hashi K, Aoki N, Fukuhara S, Hashimoto N. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med. 2012. 366: 2474-82
12. Jaimovich SG, Guevara M, Pampin S, Jaimovich R, Gardella JL. Neurosurgical planning using osirix software. Surg Neurol Int. 2014. 5: S267-71
13. Krisht AF. Transcavernous approach to diseases of the anterior upper third of the posterior fossa. Neurosurg Focus. 2005. 19: 1-10
14. Krisht AF, Krayenbuhl N, Sercl D, Bikmaz K, Kadri PA. Results of microsurgical clipping of 50 high complexity basilar apex aneurysms. Neurosurgery. 2007. 60: 242-50
15. Kuruoglu E, Aydin K, Marangoz A, Cokluk C. The Contribution of Three-Dimensional Computerized Tomographic Angiography in the Head Positioning of the Patients with Middle Cerebral Artery Aneurysms. Turk Neurosurg. 2015. 25: 793-5
16. Lehecka M LA, Hernesniemi J.editorsMicroneurosurgery Basics and Tricks. KG/Germany: Druckerei Hohl Gmbh & Co; 2011. p.
17. Mandel M, Amorim R, Paiva W, Prudente M, Teixeira MJ, Andrade AF. 3D preoperative planning in the ER with OsiriX(R): When there is no time for neuronavigation. Sensors. 2013. 13: 6477-91
18. Molyneux AJ, Kerr RS, Yu LM, Clarke M, Sneade M, Yarnold JA. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: A randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005. 366: 809-17
19. Mooney MA, Kalani MY, Nakaji P, Albuquerque FC, McDougall CG, Spetzler RF. Long-term Patient Outcomes After Microsurgical Treatment of Blister-Like Aneurysms of the Basilar Artery. Neurosurgery. 2015. 11: 387-93
20. Peerless SJ DC, Wilkins RH, Rengachary SS.editors. Posterior circulation aneurysms. Neurosurgery. New York: McGraw-Hill; 1985. p. 1422-37
21. Poblete T, Jiang X, Komune N, Matsushima K, Rhoton AL. Preservation of the nerves to the frontalis muscle during pterional craniotomy. J Neurosurg. 2015. 122: 1274-82
22. Rhoton AL. Aneurysms. Neurosurgery. 2002. 51: S121-158
23. Rhoton AL. Tentorial incisura. Neurosurgery. 2000. 47: S131-53
24. Rosset A, Spadola L, Ratib O. OsiriX: An open-source software for navigating in multidimensional DICOM images. J Digital Imaging. 2004. 17: 205-16
25. Sabuncuoglu H, Jittapiromsak P, Cavalcanti DD, Spetzler RF, Preul MC. Accessing the basilar artery apex: Is the temporopolar transcavernous route an anatomically advantageous alternative?. Skull Base. 2011. 21: 23-30
26. Saeki N, Rhoton AL. Microsurgical anatomy of the upper basilar artery and the posterior circle of Willis. J Neurosurg. 1977. 46: 563-78
27. Sekhar LN, Tariq F, Morton RP, Ghodke B, Hallam DK, Barber J. Basilar tip aneurysms: A microsurgical and endovascular contemporary series of 100 patients. Neurosurgery. 2013. 72: 284-98
28. Spetzler RF, McDougall CG, Albuquerque FC, Zabramski JM, Hills NK, Partovi S. The Barrow Ruptured Aneurysm Trial: 3-year results. J Neurosurg. 2013. 119: 146-57
29. Spetzler RF, McDougall CG, Zabramski JM, Albuquerque FC, Hills NK, Russin JJ. The Barrow Ruptured Aneurysm Trial: 6-year results. J Neurosurg. 2015. 123: 609-17
30. Spiriev T, Ebner FH, Hirt B, Shiozawa T, Gleiser C, Tatagiba M. Fronto-temporal branch of facial nerve within the interfascial fat pad: Is the interfascial dissection really safe?. Acta Neurochir. 2016. 158: 527-32
31. Wiebers DO, Whisnant JP, Huston J, Meissner I, Brown RD, Piepgras DG. Unruptured intracranial aneurysms: Natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003. 362: 103-10
32. Yasargyl M. .