- Department of Neurosurgery, Medical University of Gdansk, Gdansk, Poland
- Department of Neurosurgery, Medical University of Lodz, Lodz, Poland
- Institute of Medicine, Opole University, Opole, Poland,
- Division of Neurosurgery, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, United States,
- Department of Neurosurgery, Pomeranian Medical University, Szczecin,
- Department of Neurosurgery, Polish Mother Memorial Research Institute, Poland
- Department of Neurosurgery and Neuro-oncology, Medical University of Lodz, Lodz, Poland.
Correspondence Address:
Rafael A. Vega, Division of Neurosurgery, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, United States.
DOI:10.25259/SNI_167_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Samuel D. Pettersson1, Redwan Jabbar2, Mirosława Popławska3, Aleksander Och1, Eduardo Orrego-Gonzalez4, Tomasz Klepinowski5, Michał Krakowiak1, Leszek Sagan5, Maciej Radek2, Krzysztof Zakrzewski6, Emilia Nowoslawska6, Katarzyna Kwiecien7, Paulina Skrzypkowska1, Tomasz Szmuda1, Grzegorz Miękisiak3, Rafael A. Vega4. Telovelar versus transvermian approach to tumors of the fourth ventricle and their impact on postoperative neurological complications: A multicenter study. 07-Apr-2023;14:124
How to cite this URL: Samuel D. Pettersson1, Redwan Jabbar2, Mirosława Popławska3, Aleksander Och1, Eduardo Orrego-Gonzalez4, Tomasz Klepinowski5, Michał Krakowiak1, Leszek Sagan5, Maciej Radek2, Krzysztof Zakrzewski6, Emilia Nowoslawska6, Katarzyna Kwiecien7, Paulina Skrzypkowska1, Tomasz Szmuda1, Grzegorz Miękisiak3, Rafael A. Vega4. Telovelar versus transvermian approach to tumors of the fourth ventricle and their impact on postoperative neurological complications: A multicenter study. 07-Apr-2023;14:124. Available from: https://surgicalneurologyint.com/surgicalint-articles/12245/
Abstract
Background: Tumors of the fourth ventricle are exceedingly rare; however, such lesions are formidable due to the severe postoperative neurological complications (pNCs) which often occur. The adoption of the telovelar approach over the transvermian was created to supposedly mitigate the pNCs; however, there is a lack of sufficient data supporting this theory.
Methods: Records from six hospitals were reviewed for patients surgically treated for a single tumor within the 4th ventricle from 2016 to 2022. The pNCs which had 10 or more occurrences among the patients were individually assessed as the dependent variable in a binary logistic regression model against covariates which included the surgical approach.
Results: This study of 67 patients confirms no significant differences in risk for pNCs between the transvermian and telovelar approach. Rather, multivariate analysis identified neurophysiological monitoring (IONM) as a protective factor for postoperative speech and swallowing defects (odds ratio [OR]: 0.076, 95% confidence interval [CI] 0.011–0.525). Furthermore, intraoperative external ventricular drainage (EVD) was a protective factor for postoperative gait and focal motor defects (OR: 0.075, 95% CI 0.009–0.648) and for postoperative hydrocephalus (OR: 0.020, 95% CI 0.002–0.233). A univariate meta-analysis pooling the present study’s patients and an additional 304 patients from the three additional studies in the literature confirms no significant differences in risk between the transvermian and telovelar approach for pNCs.
Conclusion: Intraoperative adjuncts including IONM and EVD may play a significant role in the postoperative outcome. Despite the present study’s sample size being a major limitation, the findings may provide great value to neurosurgeons given the scarcity of the current literature.
Keywords: Complications, Fourth ventricle, Telovelar, Transvermian, Tumor
INTRODUCTION
Tumors of the fourth ventricle are exceedingly rare; however, such lesions are considered formidable and pose a significant challenge to most neurosurgeons. Surrounded by vital structures of the brainstem, the available options to surgically approach such lesions are limited, while the risk of complications is high. The present literature shows that the prevalence of postoperative neurological complications (pNCs) following surgical treatment is fairly high. These include cerebellar mutism syndrome (CMS), gait disturbances, cranial nerve defects, and visual impairment as some of the most common and their prevalence rates are listed as follows: 20.5%,[
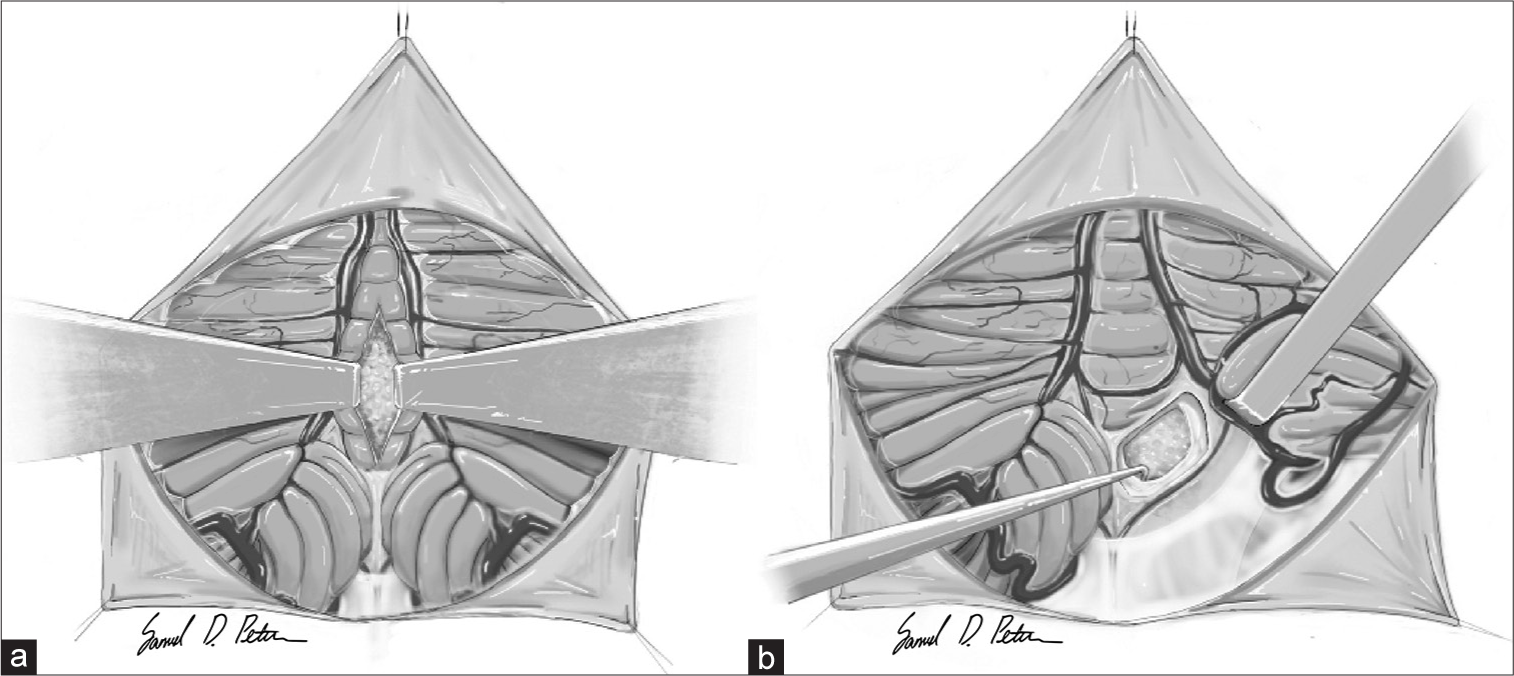
Figure 1:
A basic illustration of the midline approach types. (a) The transvermian approach: a midline incision of the inferior half of the cerebellar vermis and retracting the two halves exposing a tumor in the fourth ventricle. (b) The (unilateral) telovelar approach: lateral retraction of the right cerebellar tonsil and opening the tela choroidea exposing a tumor in the fourth ventricle.
The current literature is scarce regarding the risks and benefits of utilizing the telovelar over the transvermian approach and to the best of our knowledge, only three studies exist assessing the two approach types against postoperative complications.[
MATERIALS AND METHODS
Data extraction
This study was registered under local research and development protocols, and ethical approval was granted from the institutional review board. Patients diagnosed with a FVT who underwent surgical resection between January 2016 and September 2022 at 6 neurosurgical centers (Gdańsk, Poland; Olsztyn, Poland; Wrocław, Poland; Szczecin, Poland; and 2 in Łódź, Poland) were included in this study. Any patients with additional intracranial tumors located elsewhere were excluded from the study. For each patient, demographic data, risk factors, reasons for treatment, clinical presentation, and tumor characteristics on preoperative imaging were collected. Data regarding the surgical approach were obtained from detailed operative reports. The selection of surgical approach was based on surgeon preference due to the lack of an official guideline and the extent of resection was determined from the review of the postoperative magnetic resonance imaging (MRI) and computed tomography (CT) scans. Gross-total resection was defined as complete tumor resection with no evidence of residual tumor on postoperative MRI or CT. Postoperative neurological outcomes were reviewed using in-patient hospital records and outpatient clinic notes. Both new and worsening deficits were counted as complications. Postoperative neurological function was assessed before discharge.
Search strategy selection criteria
To conduct a meta-analysis, the screening process was performed according to the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analyses. PubMed, Web of Science, and Scopus databases were used to retrieve studies from inception to September 2022, without language limits. The following keywords were used in all three databases: (fourth_ventricle* OR fourth_ventricular) AND (tumor* OR tumor*) AND (transvermian OR telovelar OR transcerebellomedullary_fissure OR approach OR treatment). The search was performed independently by two authors (Samuel D. Pettersson and Eduardo Orrego-Gonzalez), and another author (Rafael A. Vega) arbitrated any disagreements on inclusion or exclusion of the studies.
The first phase of screening included assessing the article titles and abstracts for three requirements: written in the English language, involving patients with fourth ventricle tumors, and reporting the telovelar or transvermian approaches. The studies that passed the first screening were reassessed in a second screening phase. The studies were required to provide extractable data. Any studies that involved fewer than ten patients and/or consisted of patients who underwent only one of the approach types were excluded from the study.
Statistical analysis
Given that Ferguson et al.[
Regarding the meta-analysis, the approach used was treated as a dichotomous variable and was pooled into an overriding OR and 95% confidence interval (CI) to identify risk factors for each pNCs that were reported at least twice in the literature. If a study failed to provide standard deviations, the value was calculated using standard errors, CIs, t-values, or P-values that relate to the differences between means in two groups.[
RESULTS
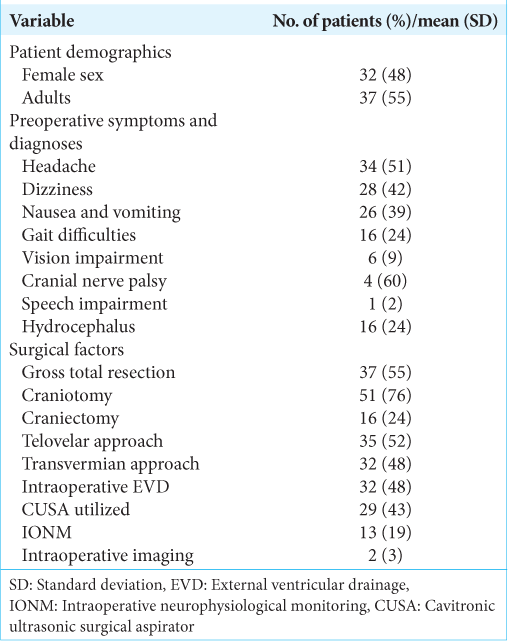
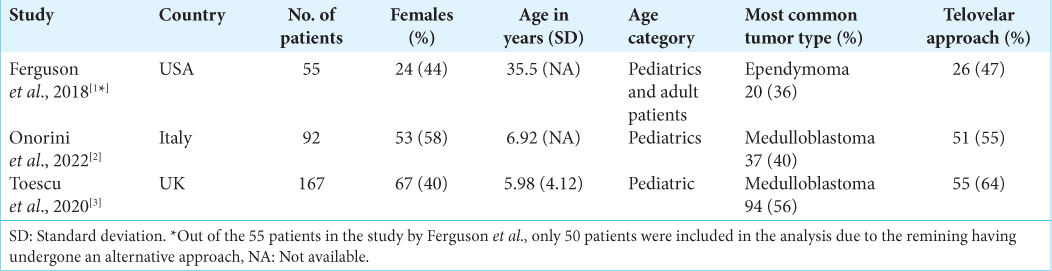
A total of 79 patients adhered to the selection criteria. Out of the 79, a total of 12 were excluded as these patients underwent a non-midline approach (a trans-cerebellar approach) due to the tumor having invaded up to the surface of the cerebellar hemisphere. Thus, 67 patients were included in the study. About 47.8% of the included patients were female and 44.8% were pediatrics (<18-years-old) [
All surgical procedures began with exposing the posterior fossa through a craniotomy (76.1%) or craniectomy (23.9%), following the telovelar (52.2%) or transvermian approach (47.8%) to expose the fourth ventricle. An external ventricular drain was placed prior to the operation in almost half the patients (47.8%) and intraoperative neurophysiological monitoring (IONM) was performed in 19.4% of the procedures. Despite gross total resection being the goal for each patient, such a result was only achieved in 55.2% of the patients.
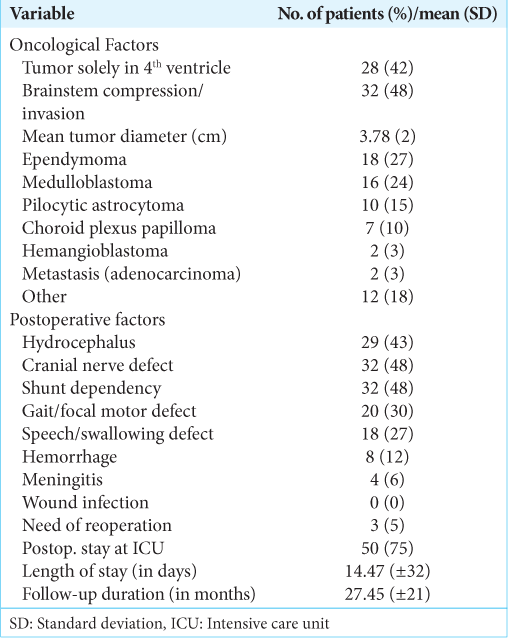
The tumor type was reported postoperative with the most common being medulloblastoma (23.9%), ependymoma (26.9%), pilocytic astrocytoma (14.9%), and choroid plexus papilloma (10.4%) [
Risk factors for postoperative neurological complications
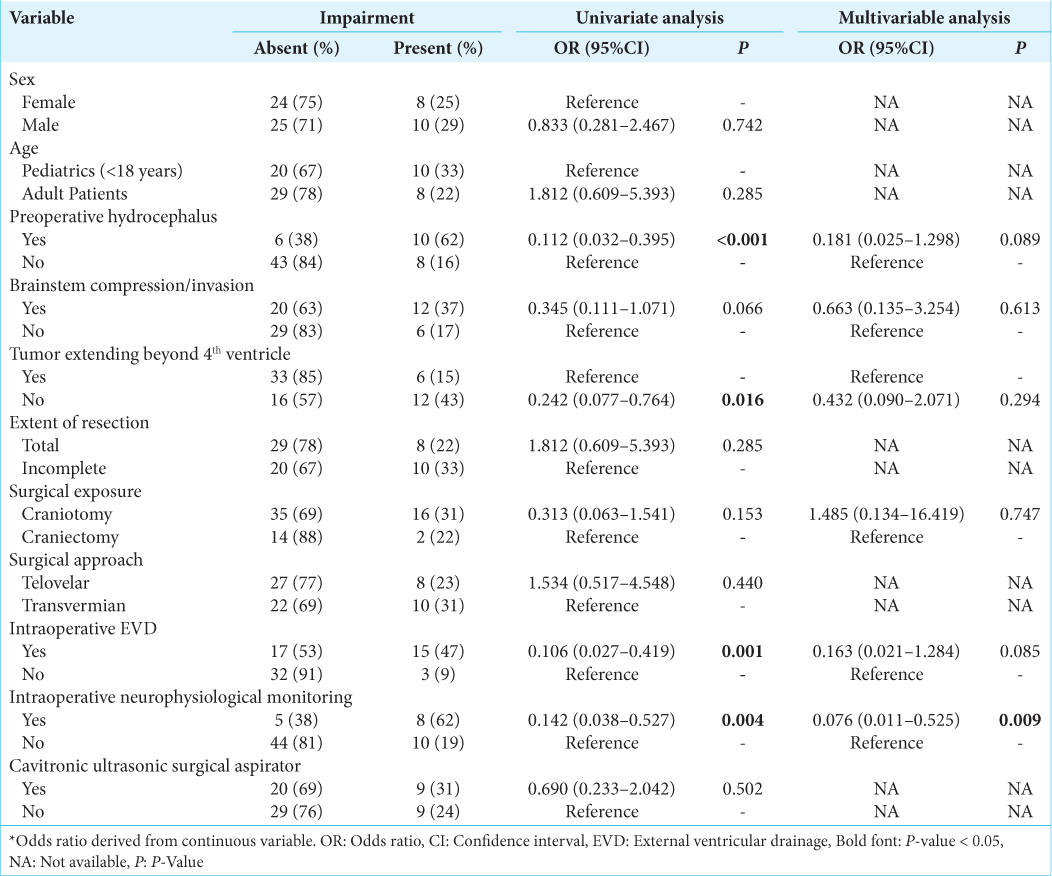
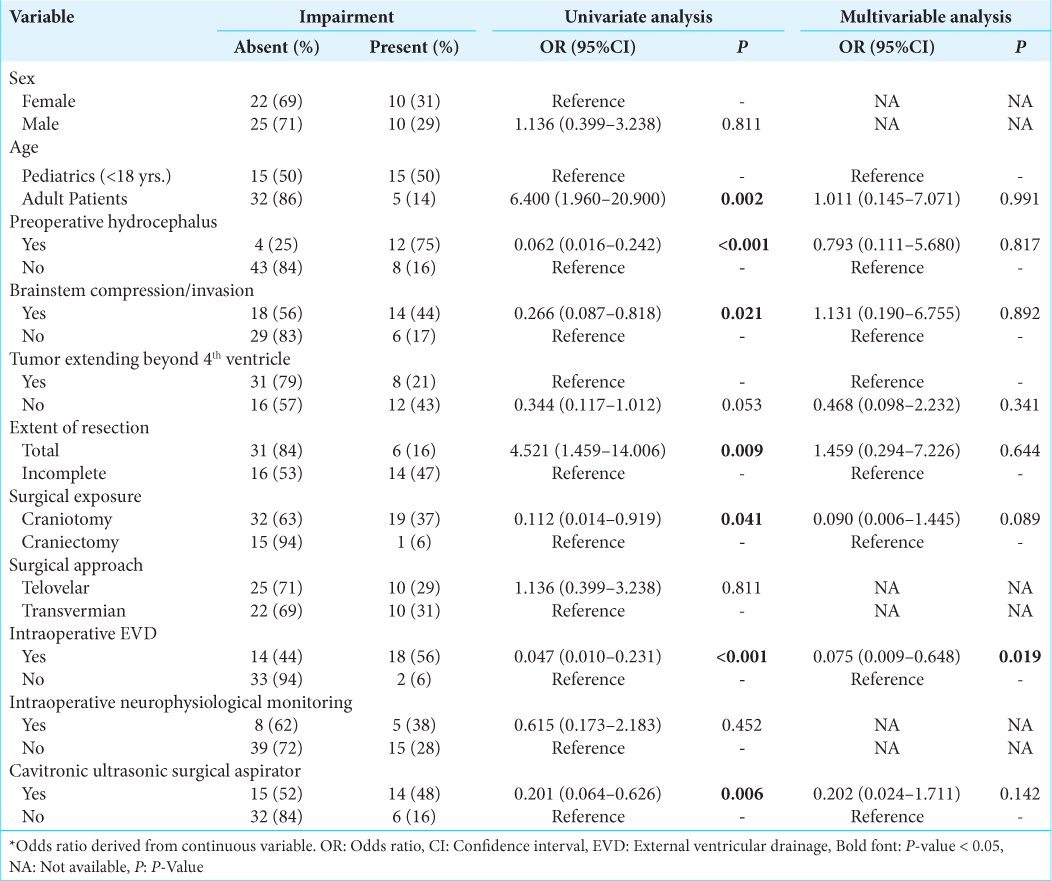
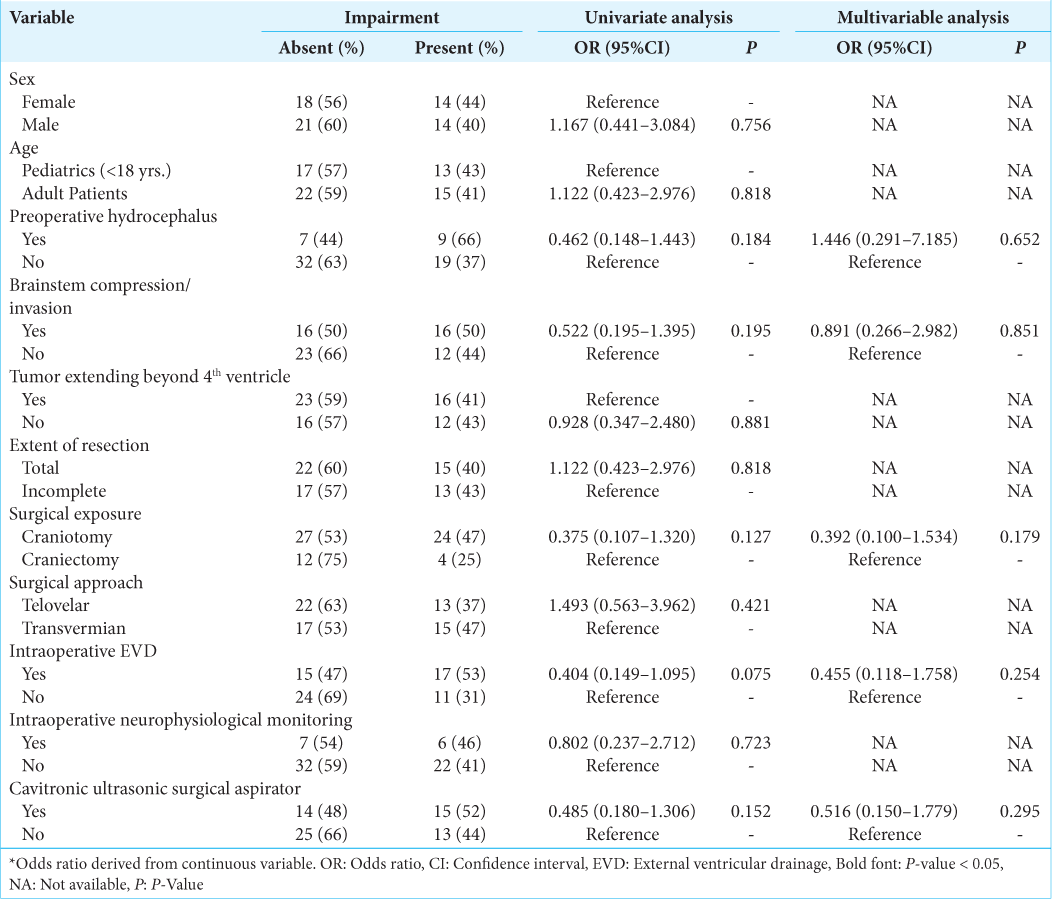
Four pNCs had 10 or more occurrences which allowed them to be inputted into regression analysis. For postoperative cranial nerve defects, neither the univariate nor multivariate analysis yielded no statistically significant predictors [Supplementary
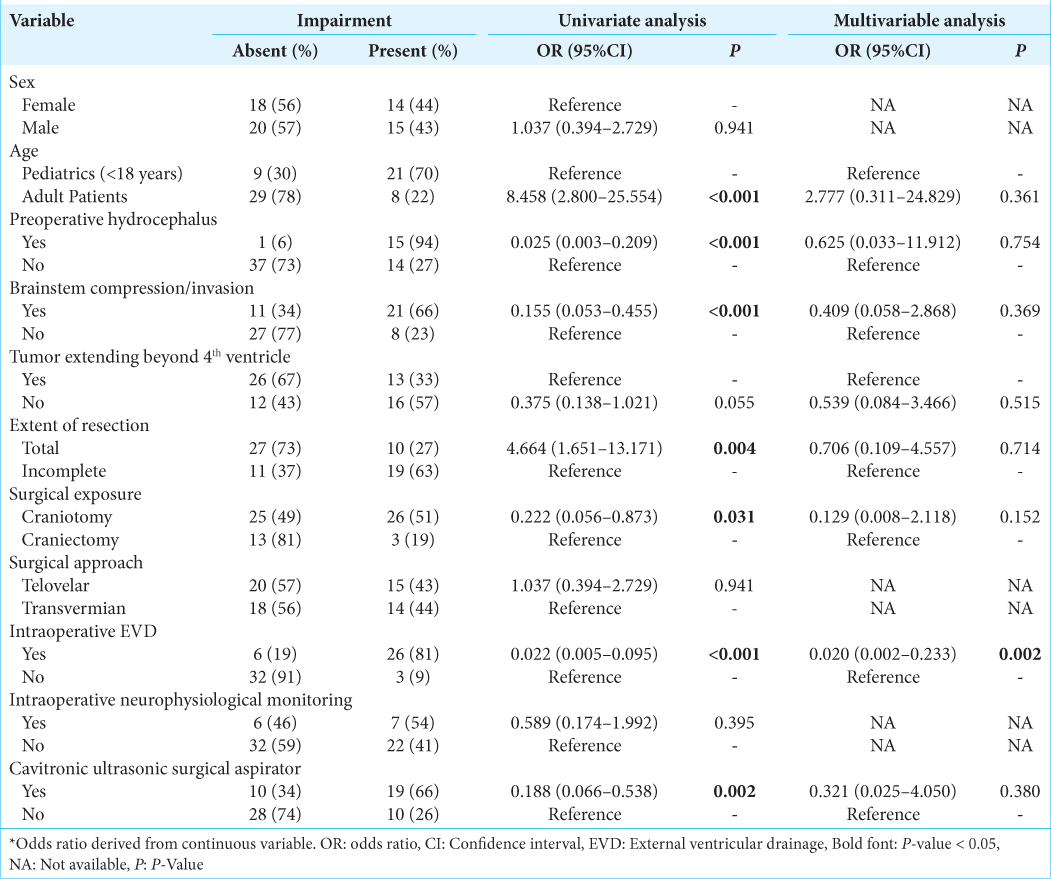
For postoperative gait/focal motor defects, univariate analysis confirmed adult age, preoperative hydrocephalus, brainstem compression, complete resection, surgical exposure, intraoperative EVD, and using a cavitronic ultrasonic surgical aspirator (CUSA) as significant predictors [
For postoperative hydrocephalus, univariate analysis confirmed adult age, preoperative hydrocephalus, brainstem compression, complete resection, surgical exposure, intraoperative EVD, and using a CUSA as significant predictors [
Meta-analysis
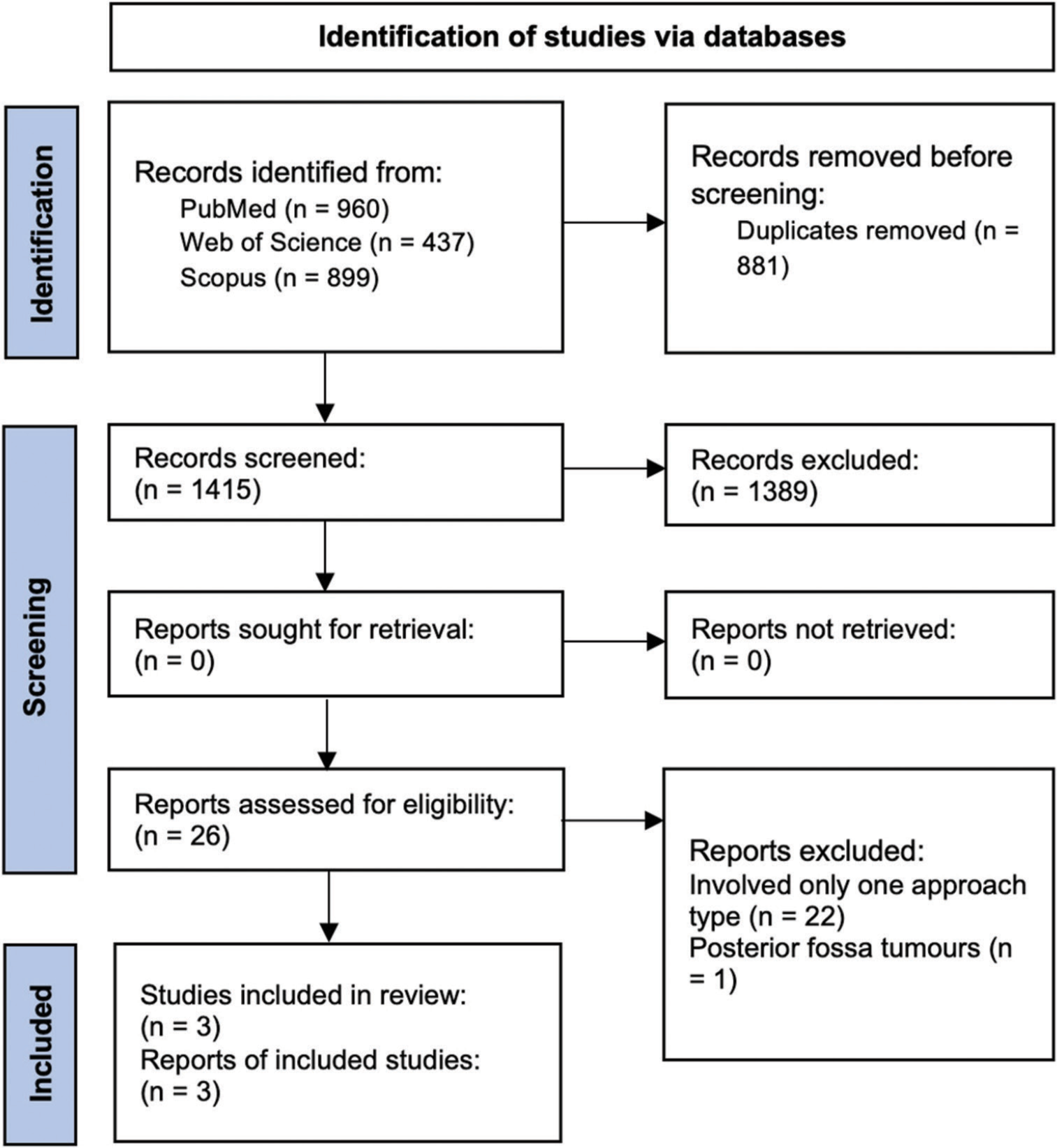
A total of 1415 unduplicated records were identified. Titles and abstracts were assessed for relevance and 1389 records were removed. Out of the remaining 26 records, 22 involved only one surgical approach type, and 1 did not involve solely fourth ventricle tumors [Supplementary
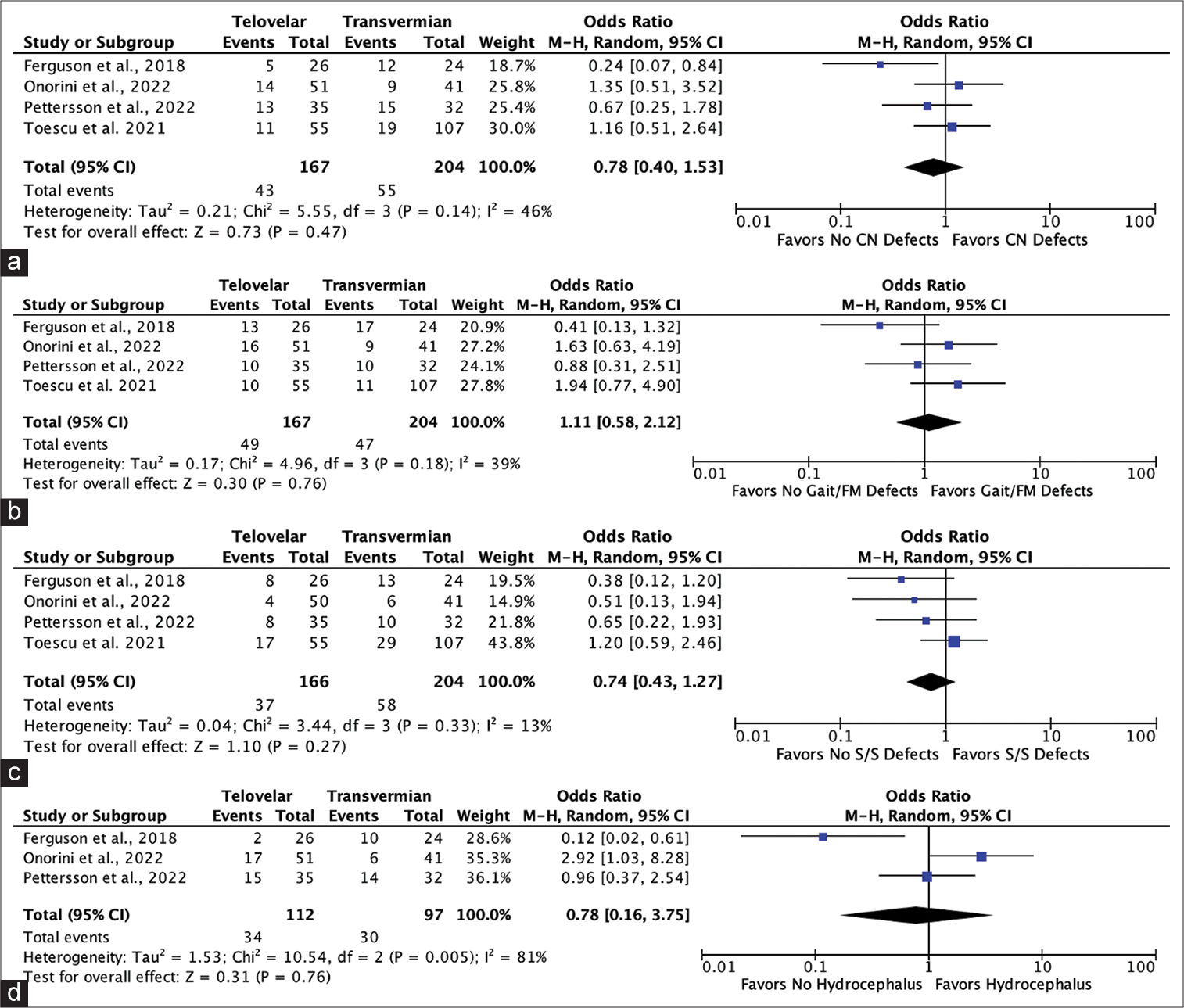
Figure 2:
Forest plots showing no significant risk between utilizing the telovelar over the transvermian for (a) postoperative cranial nerve defects, (b) gait/focal motor defects, (c) postoperative speech/ swallowing defects, or (d) postoperative hydrocephalus following fourth ventricle tumor resection. CN: Cranial nerve, FM: Focal motor, S/S: Speech/swallowing, CI: Confidence interval, M-H: Mantel-haenszel, P: P-value, df: degrees of freedom, Z: Cohen’s D effect size.
DISCUSSION
The optimal way to approach tumors of the fourth ventricle is controversial. Furthermore, given the rarity of these tumors, the available studies on methods of management are scarce which has resulted in the overall treatment of such tumors being heavily opinion-based in regard to the various adjuncts one can utilize including neurophysiologic monitoring and intraoperative imaging. The telovelar approach has been shown to become the more popular choice over the past decades from our experiences and from the results from Toescu et al.[
The telovelar and transvermian approaches offer their own unique advantages to different regions of the fourth ventricle. Thus, not all fourth ventricle tumors are ideal candidates for the telovelar approach when compared to the transvermian, and vice versa. The increasing trend shown by Toescu et al.[
Methods of mitigating the risk for pNCs
Despite no significant difference in risk between utilizing the telovelar over the transvermian approach, the postoperative complication rates remain concerningly high. Thus, it is critical to discuss the additional factors identified by the present study which were shown to independently mitigate the risks for pNCs as such a discussion currently does not exist in the literature.
IONM
The use of IONM is an opinion-based decision from the surgeon and thus, each clinic varies in the frequency of the adjunct’s incorporation into the surgery. Choosing to use IONM will require a longer operating room preparation time and adds additional challenges for the surgeon and the anesthesia team. Factors such as maintaining the body core temperature above 36.5°C for sufficient amplitudes from motor evoked potentials, keeping the electrodes fixed to their structures throughout the procedure, and having to use an intravenous anesthetic rather than an inhaled are some of the many added challenges. Therefore, this is likely the reason why only 33% of the patients from Ferguson et al.[
EVD
Performing EVD before surgery is controversial. Older studies from the 70’s and 80’s found that preoperative EVD was overall positive as it was shown to be associated with the lower mortality rates, less deterioration, and immediate resolution of papilledema when compared to no EVD.[
Limitations
The present study has several limitations. Aside from being retrospective in nature, the sample size is small given the rarity of fourth ventricle tumors. Therefore, having the room to control the patients who had any of the pNCs before surgery to increase the quality of our results could not be done without sacrificing the ability to analyze risk factors for the few pNCs which had lower occurrences. Furthermore, pediatrics and adult patients were mixed in our analysis and performing subgroup analyses for the two age groups would have been ideal to yield stronger results. However, the limited sample size prevented the ability to perform such an analysis. It is also worth to point out that brainstem compression/ invasion, although non-significant, yields an OR indicating the variable as a protective factor for two of the pNCs in multivariate analysis. This, however, does not make intuitive sense and may suggest that a confounder could be affecting our analysis. To improve on the risks of bias in the present study, we encourage future investigators with a larger cohort of patients to perform a propensity score analysis to reduce selection bias as it is the optimal method to carry out such a study. A minimum sample size of 200 patients would be required. Regarding our multivariate analysis, three independent protective factors for various pNCs were identified, however, given their wide CIs, we advise specialists to interpret the results with caution as the regression models may be overfitted. Overall, we encourage future investigators to improve upon the present study by accounting for its limitations, and by assessing the replicability of the significant findings.
CONCLUSION
The present study confirms no significant differences in risks for pNCs between the telovelar and transvermian approaches to tumors of the fourth ventricle. Rather, the intraoperative adjuncts including neurophysiological monitoring and EVD may play a significant role in the postoperative outcome. Since both the telovelar and transvermian approach offer their own unique advantages to tumors situated in various locations of the fourth ventricle, our findings suggest that surgeons should use the surgical approach which offers the greatest access to the specific target in the fourth ventricle, as forcing one’s entry into regions of the ventricle which are not easily accessible with one approach when compared to the latter may rather risk damaging the adjacent structures, and possibly, increase the risk for neurological complications.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
SUPPLEMENTARY FIGURE
Supplementary Figure 1:
PRISMA flowchart of the scientific literature search and study selection. Data added to the PRISMA template (from Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021;372:n71) under the terms of the Creative Commons Attribution License. n: number of references
SUPPLEMENTARY TABLES
References
1. 7.7.3.2 Obtaining Standard Deviations from Standard Errors and Confidence Intervals for Group Means. Available from: https://www.handbook-5-1.cochrane.org/chapter_7/7_7_3_2_obtaining_standard_deviations_from_standard_errors_and.htm [Last accessed on 2022 Sep 25].
2. Albright L, Reigel DH. Management of hydrocephalus secondary to posterior fossa tumors. J Neurosurg. 1977. 46: 52-5
3. Anania P, Battaglini D, Balestrino A, D’Andrea A, Prior A, Ceraudo M. The role of external ventricular drainage for the management of posterior cranial fossa tumours: A systematic review. Neurosurg Rev. 2020. 44: 1243-53
4. Ashida R, Nazar N, Edwards R, Teo M. Cerebellar Mutism syndrome: An overview of the pathophysiology in relation to the cerebrocerebellar anatomy, risk factors, potential treatments, and outcomes. World Neurosurg. 2021. 153: 63-74
5. Atallah A, Rady MR, Kamal HM, El-Mansy N, Alsawy MF, Hegazy A. Telovelar approach to pediatric fourth ventricle tumors: Feasibility and outcome. Turk Neurosurg. 2019. 29: 497-505
6. Cobourn K, Marayati F, Tsering D, Ayers O, Myseros JS, Magge SN. Cerebellar Mutism syndrome: Current approaches to minimize risk for CMS. Childs Nerv Syst. 2020. 36: 1171-9
7. Deshmukh VR, Figueiredo EG, Deshmukh P, Crawford NR, Preul MC, Spetzler RF. Quantification and comparison of telovelar and transvermian approaches to the fourth ventricle. Neurosurgery. 2006. 58: ONS-202-6 discussion ONS-206-7
8. Eissa EM. The role of the telovelar approach in fourth ventricular surgery: A new perspective. Turk Neurosurg. 2018. 28: 523-9
9. Ferguson SD, Levine NB, Suki D, Tsung AJ, Lang FF, Sawaya R. The surgical treatment of tumors of the fourth ventricle: A single-institution experience. J Neurosurg. 2017. 128: 339-51
10. Foreman P, McClugage S, Naftel R, Griessenauer CJ, Ditty BJ, Agee BS. Validation and modification of a predictive model of postresection hydrocephalus in pediatric patients with posterior fossa tumors. J Neurosurg Pediatr. 2013. 12: 220-6
11. Hosmer DW, Lemeshow S, Sturdivant RX, editors. Applied Logistic Regression. United States: Wiley; 2013. p. 1-510
12. Marx S, Reinfelder M, Matthes M, Schroeder HW, Baldauf J. Frequency and treatment of hydrocephalus prior to and after posterior fossa tumor surgery in adult patients. Acta Neurochi (Wien). 2018. 160: 1063-71
13. Matsushima T, Fukui M, Inoue T, Natori Y, Baba T, Fujii K. Microsurgical and magnetic resonance imaging anatomy of the cerebello-medullary fissure and its application during fourth ventricle surgery. Neurosurgery. 1992. 30: 325-30
14. Onorini N, Spennato P, Orlando V, Savoia F, Calì C, Russo C. The clinical and prognostic impact of the choice of surgical approach to fourth ventricular tumors in a single-center, single-surgeon cohort of 92 consecutive pediatric patients. Front Oncol. 2022. 12: 821738
15. Pettersson SD, Kitlinski M, Miekisiak G, Ali S, Krakowiak M, Szmuda T. Risk factors for postoperative cerebellar Mutism syndrome in pediatric patients: A systematic review and meta-analysis. J Neurosurg Pediatr. 2021. 29: 467-75
16. Raimondi AJ, Tomita T. Hydrocephalus and infratentorial tumors: Incidence, clinical picture, and treatment. J Neurosurg. 1981. 55: 174-82
17. Rajesh BJ, Rao BR, Menon G, Abraham M, Easwer HV, Nair S. Telovelar approach: Technical issues for large fourth ventricle tumors. Childs Nerv Syst. 2007. 23: 555-8
18. Tamburrini G, Pettorini BL, Massimi L, Caldarelli M, Di Rocco C. Endoscopic third ventriculostomy: The best option in the treatment of persistent hydrocephalus after posterior cranial fossa tumour removal?. Childs Nerv Syst. 2008. 24: 1405-12
19. Tanriover N, Ulm AJ, Rhoton AL, Yasuda A. Comparison of the transvermian and telovelar approaches to the fourth ventricle. J Neurosurg. 2004. 101: 484-98
20. Toescu SM, Samarth G, Horsfall HL, Issitt R, Margetts B, Phipps KP. Fourth ventricle tumors in children: Complications and influence of surgical approach. J Neurosurg Pediatr. 2020. 27: 52-61
21. Won SY, Gessler F, Dubinski D, Eibach M, Behmanesh B, Herrmann E. A novel grading system for the prediction of the need for cerebrospinal fluid drainage following posterior fossa tumor surgery. J Neurosurg. 2019. 132: 296-305
22. Zaheer SN, Wood M. Experiences with the telovelar approach to fourth ventricular tumors in children. Pediatr Neurosurg. 2010. 46: 340-3