- Department of Paediatric Neurosurgery, Hospital Italiano de Buenos Aires, Buenos Aires, Argentina.
Correspondence Address:
Daniela Sol Massa, Department of Paediatric Neurosurgery, Hospital Italiano de Buenos Aires, Buenos Aires, Argentina.
DOI:10.25259/SNI_299_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Daniela Sol Massa, Nicolas Arturo Montivero, Santiago Adalberto Portillo Medina. Terminal myelocystocele: Surgical management. 03-Jun-2022;13:234
How to cite this URL: Daniela Sol Massa, Nicolas Arturo Montivero, Santiago Adalberto Portillo Medina. Terminal myelocystocele: Surgical management. 03-Jun-2022;13:234. Available from: https://surgicalneurologyint.com/surgicalint-articles/11633/
Abstract
Background: The authors describe clinical and imaging findings, surgical technique, and outcomes in myelocystocele.
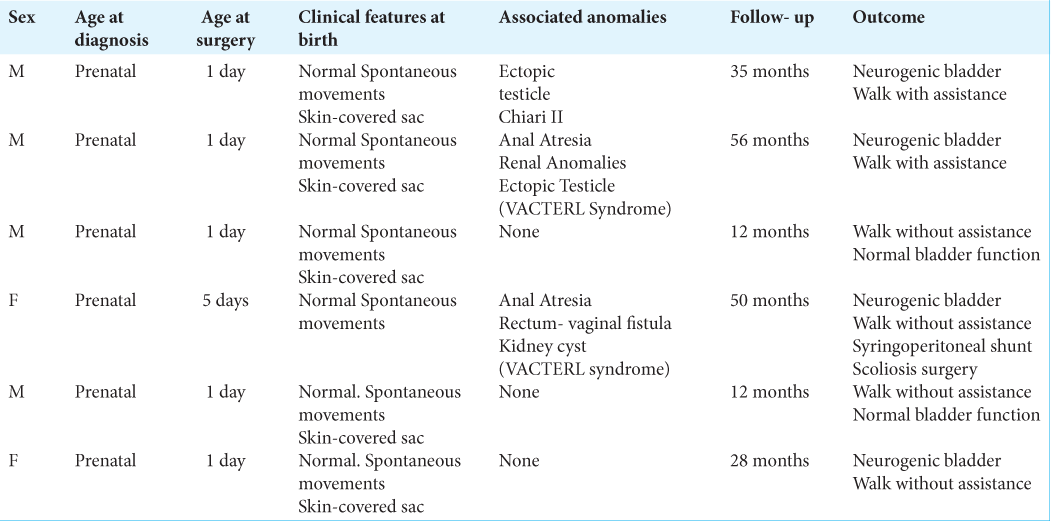
Methods: We describe a surgical procedure performed in six patients, four males and two females, with myelocystocele treated at our hospital. We review the images obtained at the time of diagnosis and after surgery. The patients’ age range was 12–56 months and had undergone surgery for terminal myelocystocele between 2015 and 2020. All patients had a large lumbar mass covered with healthy skin and presented spontaneous movements at birth. Two patients presented VACTERL syndrome.
Results: A watertight closure of the soft tissues was performed in all cases. None of the patients presented postsurgical complications, such as cerebrospinal fluid leak or infection. All the patients had undergone excision of the meningocele sacs, the tethering bands were lysed, and the filum was detethered. The mean follow-up period was 34 (12–56) months. A motor deficit was seen in 2 patients (33.3%).
Conclusion: Prenatal diagnosis and early corrective surgical intervention are recommended to prevent deterioration in neurological function. VACTERL association is a common condition and should be investigated.
Keywords: Neural tube defect, Spina bifida, Surgical technique, Terminal myelocystocele
INTRODUCTION
Myelocystocele is a rare form of occult spinal dysraphism, which consists of a cystic dilatation of the central canal of the distal spinal cord, herniated, and reflected through the posterior sac.[
MATERIALS AND METHODS
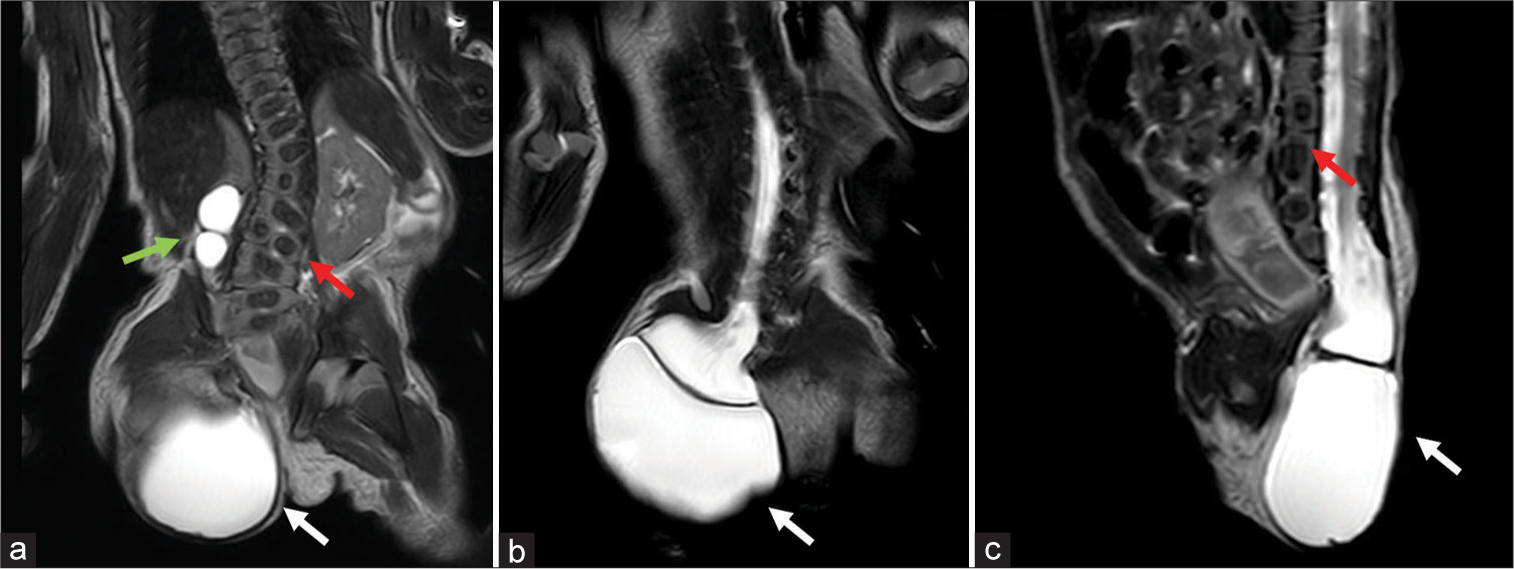
We describe the surgical procedure performed by the senior author (S.P.M.) and review the outcomes in six patients with TMC, who were operated on at (hospital’s name) between January 2015 and June 2020. Their data, retrospectively collected, include clinical and radiological features, type of surgery performed, clinical and imaging features, and outcomes. All these patients had undergone presurgical magnetic resonance imaging (MRI) [
Figure 1:
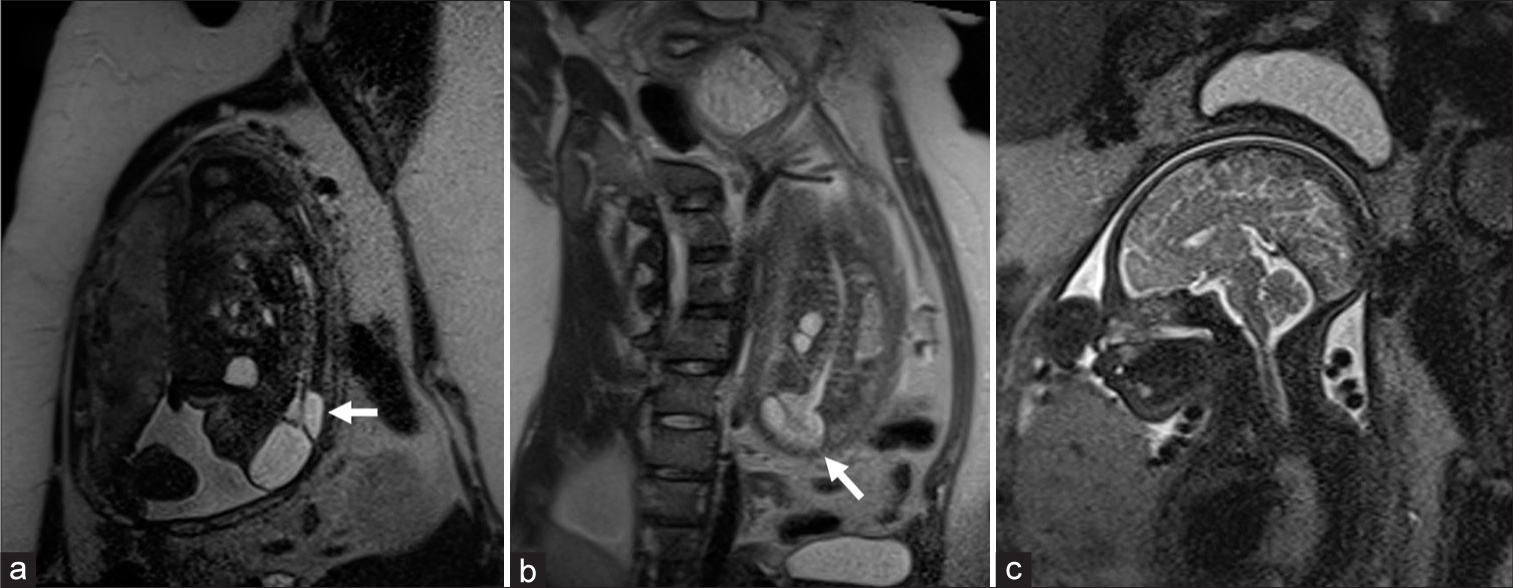
Prenatal MRI. Prenatal MRI of a fetus of 25 week of gestation. (a and b) Sagittal view. An elongated spinal cord can be seen extending dorsally out of the spinal canal, finishing reflected, and tethered in the dorsal part of dural sac, where it expands into a fluid-filled, thin-walled, trumpet-shaped cavity (trumpet-like sign , white arrows). (c) Sagittal view of head MRI without hydrocephalus or Chiari malformation.
Surgical procedure
The patients were placed in prone position. In our experience, we use an “overlapping flap” technique. After a midline vertical skin incision or a lateral semicircular incision surrounding the lumbar mass, the subcutaneous tissue was separated from the dural plane preserving the sac intact. Once the site through which the sac continued with the normal dural canal was identified, the paravertebral muscle cephalic to the sac entry was incised and dissected, and at least two sets of normal laminae were exposed and removed.
The turgidity of an intact sac had proven helpful during the initial exposure of the myelocystocele. The dura was then opened starting rostrally to the sac in the midline following the normal spinal cord separating the arachnoid adherences, sparing dorsal nerve roots, until reaching the place where distal cord is reflected in the back of the sac. Most often, the content in the cord that corresponds to the central canal was drained. In some cases, the spinal cord was rotated to one side, and when the nerve roots of each side were compared, a difference in development was seen sometimes.
Thin fibrotic bands were divided, extra tissue was excised, and conus medullaris reconstruction was done with interrupted 5-0 absorbable pial sutures. If filum terminale was identified, it was cut to detether the cord. The dura is sutured with overlapping flaps so that the terminal cord is not in contiguity with the suture of the dura, avoiding posterior tethering. The same idea is applied to the muscle flaps, with which we use barbed sutures to reduce the tension of the paraspinal muscles, and finally, the excess skin resulting from the sac that no longer exists can be resected. The vascularity and mobility of the flap are, therefore, ideal in providing a soft-tissue interposition between the skin flaps and reconstructed thecal sac or even incorporating this into the dural repair if insufficient dura is available, thus avoiding the need for xenografts or synthetic dural substitutes [
Figure 3:
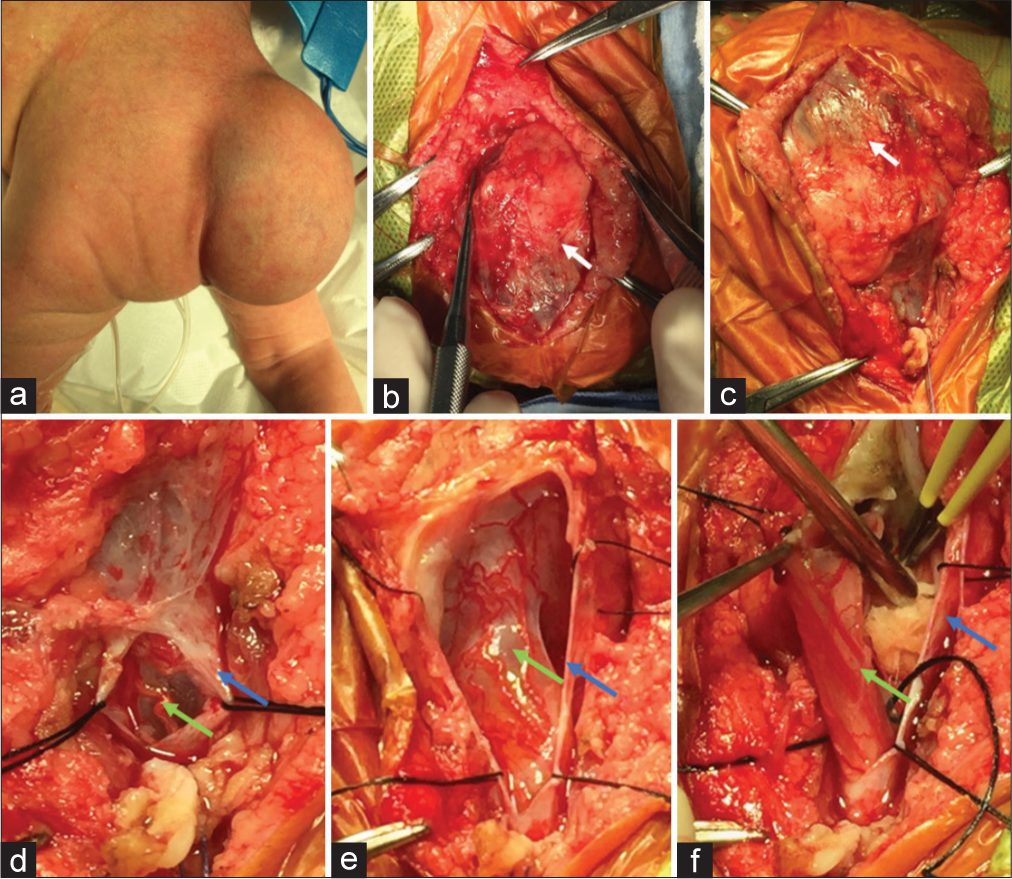
Intraoperative images. (a) Newborn back with a left-sided lumbar mass filled out of fluid. (b and c) Surgical dissection of lumbar mass. Exposition of sac of myelocystocele (white arrow). (d-f) Resection of nonfunctional neural tissue, sac closure, and nerve root preservation. After that, watertight closure is performed. Dural sac: blue arrow, neural tissue: green arrow.
Figure 4:
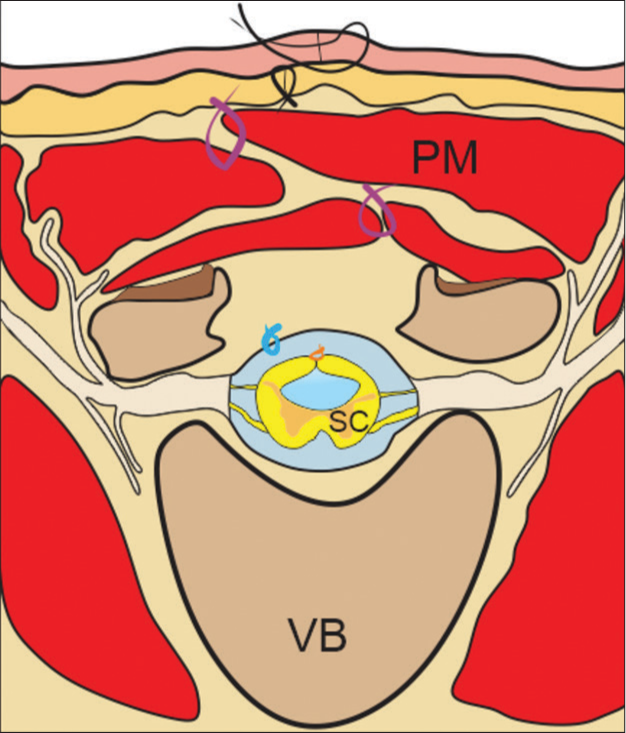
Draw of axial view at lumbar level in an open spinal tube. Axial view at lumbar level with open spinal tube, after performing an overlapped flap closure technique. Vertebral body (VB), paravertebral muscle masses (PM), and closed spinal cord (SC), orange suture: closure of the spinal cord, light blue suture: dural closure, purple suture: muscle closure, and black suture: dermal and epidermal closure.
In the immediate postoperative period, all patients were placed in prone position, and the leg end of the bed was elevated during the first 3–4 days of postoperative period. The patient received intravenous antibiotics for 2 days. The most common complication described is pseudomeningocele formation and CSF leak,[
RESULTS
All the patients were diagnosed prenatally at 12–23 weeks of pregnancy. There were 4 (66.6%) boys and 2 (33.3%) girls. All were born at full term by cesarean section. Patients carachteristics are described in
Five patients underwent surgery early after birth, one on the 5th day after birth. We made a complete preoperative multidisciplinary evaluation, due to because it is a skin covered defect, there is no urgency to surgery. In all our cases, the presumptive diagnosis was made during routine obstetric ultrasonography looking for malformations and confirmed with prenatal MRI. All preoperative MRIs show some constant (“obligatory”) features[
In all patients, we found posterior lumbosacral dimorphism, cord descent at the level of the dysraphism with the presence of myelocystocele, and associate arachnoid sac. One of the patients presented significant development of subcutaneous fat that adhered to the sac, and all the other patients had prenatal syringomyelia. Four patients had bladder dysfunction. Association with VACTERL syndrome[
The mean follow-up period was 34 (12–56) months. None of the patients presented postsurgical complications, such as CSF leak or infection.
DISCUSSION
Myelocystocele is a rare congenital malformation,[
All our patients had a skin-covered cystic mass, which is typically lumbosacral, obliterates the intergluteal cleft, and extends upward from the perineum for a variable distance. In the patients with neurological deficit at birth, the mass was slightly lateralized to the side of the leg with the worse motor deficit. It is important to know that the intergluteal fold is obliterated and distorted in myelocystocele but preserved in lipomyelomeningocele.[
MRI is the imaging study of choice for patients with neural tube malformations. In this case, it can demonstrate direct continuity of the meningocele with the subarachnoid space.[
Caudal dysgenesis is the term applied to the partial or complete absence of the sacrum, usually including the spinal cord, anorectal, and urinary malformations. The spectrum of syndromes and associations with caudal dysgenesis includes Currarino syndrome;[
The incidence of VACTERL syndrome is estimated at approximately one in 10,000–40,000 live-born infants,[
The main goal of the surgery is the release of the tethered spinal cord. The watertight closure of the soft tissues above the defect has always been a surgical challenge. Several surgical techniques have been proposed for soft-tissue closure of various spinal defects, including primary closure, split-thickness skin grafts, local skin flaps, uni- or bilateral muscle flaps, and composite flaps such as fasciocutaneous or myocutaneous flaps.[
The surgical procedure can be done using intraoperative electrophysiological monitoring,[
CONCLUSION
TMC is a congenital defect mostly dependent on secondary neurulation. The diagnosis can be made in the prenatal stage, and it is necessary to confirm it after birth. Therefore, defects such as anal atresia, bladder exstrophy, sacral agenesis, or defects in the perineal floor may also be present like VACTERL. These malformations may require surgery before the myelocystocele. The characteristics of this malformation at the level of the distal spinal cord are complex, for which the surgical treatment requires an appropriate embryological knowledge that can differentiate it from other malformations such as spinal cord lipomas or simple sacral meningoceles. CSF leakage, the most frequent complication in these surgeries, can be avoided with an adequate closure of the soft tissues. It is also important to keep in mind that these are skin-covered lesions; therefore, there is no urgency to repair them when they are in association with other major anomalies such as cloacal exstrophy, omphalocele, and imperforate anus. Instead, initial care needs to be directed toward these anomalies rather than the myelocystocele.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Publication of this article was made possible by the James I. and Carolyn R. Ausman Educational Foundation.
Conflicts of interest
The authors declare that they have no conflicts of interest. Artwork was created by Dr. Montivero using CorelDraw®.
References
1. Adra A, Cordero D, Mejides A, Yasin S, Salman F, O’Sullivan MJ. Caudal regression syndrome: Etiopathogenesis, prenatal diagnosis, and perinatal management. Obstet Gynecol Surv. 1994. 49: 508-16
2. Albright AL, Pollack IF, Andelson PD.editors. Principles and Practice of Pediatric Neurosurgery. Thieme. 2015. p.
3. Amelot A, Cretolle C, de Saint Denis T, Sarnacki S, Catala M, Zerah M. Spinal dysraphism as a new entity in VAC TE. RL syndrome, resulting in a novel acronym V.A.C.T.E.R.L.S. Eur J Pediatr. 2020. 179: 1121-9
4. Arad E, Barnea Y, Gur E, Amir A, Leshem D, Zaretski A. Paravertebral turnover flaps for closure of large spinal defects following tethered cord repair. Ann Plast. 2006. 57: 642-5
5. Brown OH, Makar KG, Ulma RM, Buchman SR, Kasten SJ, Muraszko KM. A simplified approach to myelomeningocele defect repair. Ann Plast Surg. 2021. 86: 58-61
6. Byrd SE, Harvey C, Darling CF. MR of terminal myelocystoceles. Eur J Radiol. 1995. 20: 215-20
7. Chaturvedi A, Franco A, Chaturvedi A, Klionsky NB. Caudal cell mass developmental aberrations: An imaging approach. Clin Imaging. 2018. 52: 216-25
8. Choi S, McComb JG. Long-term outcome of terminal myelocystocele patients. Pediatr Neurosurg. 2000. 32: 86-91
9. Choux M.editors. Pediatric Neurosurgery. London: Churchill Livingstone; 1999. p.
10. Emsen IM. Reconstructions with different and new techniques of large and extensive myelomeningocele defects. J Craniofac Surg. 2019. 30: 584-8
11. Espinosa-García E, Martínez-Córdoba N. OEIS complex (omphalocele-exstrophy-imperforate anus-spinal defects): A confusing syndrome case report. Case Rep. 2021. 7: 41-9
12. Kumar R, Chandra A. Terminal myelocystocele. Indian J Pediatr. 2002. 69: 1083-6
13. Kuo MF, Tsai Y, Hsu WM, Chen RS, Tu YK, Wang HS. Tethered spinal cord and VACTERL association. J Neurosurg. 2007. 106: 201-4
14. Lee JY, Kim SP, Kim SW, Park SH, Choi JW, Phi JH. Pathoembryogenesis of terminal myelocystocele: Terminal balloon in secondary neurulation of the chick embryo. Childs Nerv Syst. 2013. 29: 1683-8
15. Lynch SA, Wang Y, Strachan T, Burn J, Lindsay S. Autosomal dominant sacral agenesis: Currarino syndrome. J Med Genet. 2000. 37: 561-6
16. Mortada H, Alhablany T, Bhat TA, Al Tamimi A. Closure of a large myelomeningocele defect using the V-Y rotation advancement flap (butterfly flap): A case report and literature review. Case Rep Plast Surg Hand Surg. 2021. 8: 134-9
17. Özçelik D, Yıldız KH, İş M, Döşoğlu M. Soft tissue closure and plastic surgical aspects of large dorsal myelomeningocele defects (review of techniques). Neurosurg Rev. 2005. 28: 218-25
18. Pang D, Zovickian J, Lee JY, Moes GS, Wang KC. Terminal myelocystocele: Surgical observations and theory of embryogenesis. Neurosurgery. 2012. 70: 1383-405
19. Quong WL, Bulstrode NW, Thompson DN. The use of deepithelialized skin flap in the surgical repair of terminal myelocystoceles. Childs Nerv Syst. 2015. 31: 473-9
20. Rossi A, Cama A, Piatelli G, Ravegnani M, Biancheri R, Tortori-Donati P. Spinal dysraphism: MR imaging rationale. J Neuroradiol. 2004. 31: 3-24
21. Solomon BD. Vacterl/vater association. Orphanet J Rare Dis. 2011. 6: 1-12
22. Stevenson RE, Jones KL, Phelan MC, Jones MC, Barr M, Clericuzio C. Vascular steal: The pathogenetic mechanism producing sirenomelia and associated defects of the viscera and soft tissues. Pediatrics. 1986. 78: 451-7
23. Tandon V, Garg K, Mahapatra AK. Terminal myelocystocele: A series of 30 cases and review of the literature. Pediatr Neurosurg. 2012. 48: 229-35
24. Tortori-Donati P, Rossi A, Biancheri R, Cama A. Magnetic resonance imaging of spinal dysraphism. Top Magn Resonance Imaging. 2001. 12: 375-409
25. Ulusoy MG, Koçer U, Sungur N, Karaaslan Ö, Kankaya Y, Özdemir R. Closure of meningomyelocele defects with bilateral modified VY advancement flaps. Ann Plast Surg. 2005. 54: 640-4