- Department of Neurosurgery, Barrow Neurological Institute, West Thomas Road, Phoenix, Arizona,
- Department of Neurosurgery, Loma Linda University School of Medicine, California, United States.
Correspondence Address:
Miguel Angel Lopez-Gonzalez
Department of Neurosurgery, Loma Linda University School of Medicine, California, United States.
DOI:10.25259/SNI_175_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Xiaochun Zhao, Mohamed Labib, Dinesh Ramanathan, Timothy Marc Eastin, Minwoo Song, Andrew S. Little, Mark C. Preul, Michael T. Lawton, Miguel Angel Lopez-Gonzalez. The anterior incisural width as a preoperative indicator for intradural space evaluation: An anatomical investigation. 25-Jul-2020;11:207
How to cite this URL: Xiaochun Zhao, Mohamed Labib, Dinesh Ramanathan, Timothy Marc Eastin, Minwoo Song, Andrew S. Little, Mark C. Preul, Michael T. Lawton, Miguel Angel Lopez-Gonzalez. The anterior incisural width as a preoperative indicator for intradural space evaluation: An anatomical investigation. 25-Jul-2020;11:207. Available from: https://surgicalneurologyint.com/surgicalint-articles/10157/
Abstract
Background: The opticocarotid triangle (OCT) and the carotico-oculomotor triangle (COT) are two anatomical triangles used in accessing the interpeduncular region. Our objective is to evaluate if the anterior incisural width (AIW) is an indicator to predict the intraoperative exposure through both triangles.
Methods: Twenty sides of 10 cadaveric heads were dissected and analyzed. The heads were divided into the following: Group A – narrow anterior incisura and Group B – wide anterior incisura – using 26.6 mm as a cutoff distance of the AIW. Subsequently, the area of the COT and the OCT in the transsylvian approach was measured, along with the maximum widths through the two trajectories in modified superior transcavernous approach.
Results: The COT in the wide group was shown to have a significantly larger area compared with the COT in the narrow group (38.4 ± 12.64 vs. 58.3 ± 15.72 mm, P P = 0.20), the maximum width of the OCT (6.6 ± 1.89 vs. 6.5 ± 1.38 mm, P = 1.00), or the maximum width of the COT (11.7 ± 2.06 vs. 12.2 ± 2.32 mm, P = 0.50). Clinical cases were included.
Conclusion: An AIW
Keywords: Anterior incisural width, Carotico-oculomotor triangle, Opticocarotid triangle
INTRODUCTION
The opticocarotid triangle (OCT)[
In the tentorial incisura, the junction of the anterior petroclinoid ligament and the posterior petroclinoid ligament (JAPPL) is an identifiable landmark on MRI (T1WI, T2WI, or fast imaging employing steady-state acquisition), along with being a less ideal visualization on CTA. The distance between the JAPPL on both sides represents the anterior incisural width (AIW).[
We conducted a quantitative study to investigate the relationship of the AIW and the areas of the OCT and COT, which can be helpful in evaluating the exposure acquired through either triangle in addition to determining if a transcavernous approach should be selected.
MATERIALS AND METHODS
Surgical approaches
Twenty sides of 10 embalmed cadaveric heads were investigated. The head was fixed on a Mayfield head holder, turned about 30° laterally and tilted about 15° toward the floor, keeping the malar eminence at the highest point. A standard orbitozygomatic craniotomy was performed with the lateral wall and the roof of the orbit removed [
Figure 1:
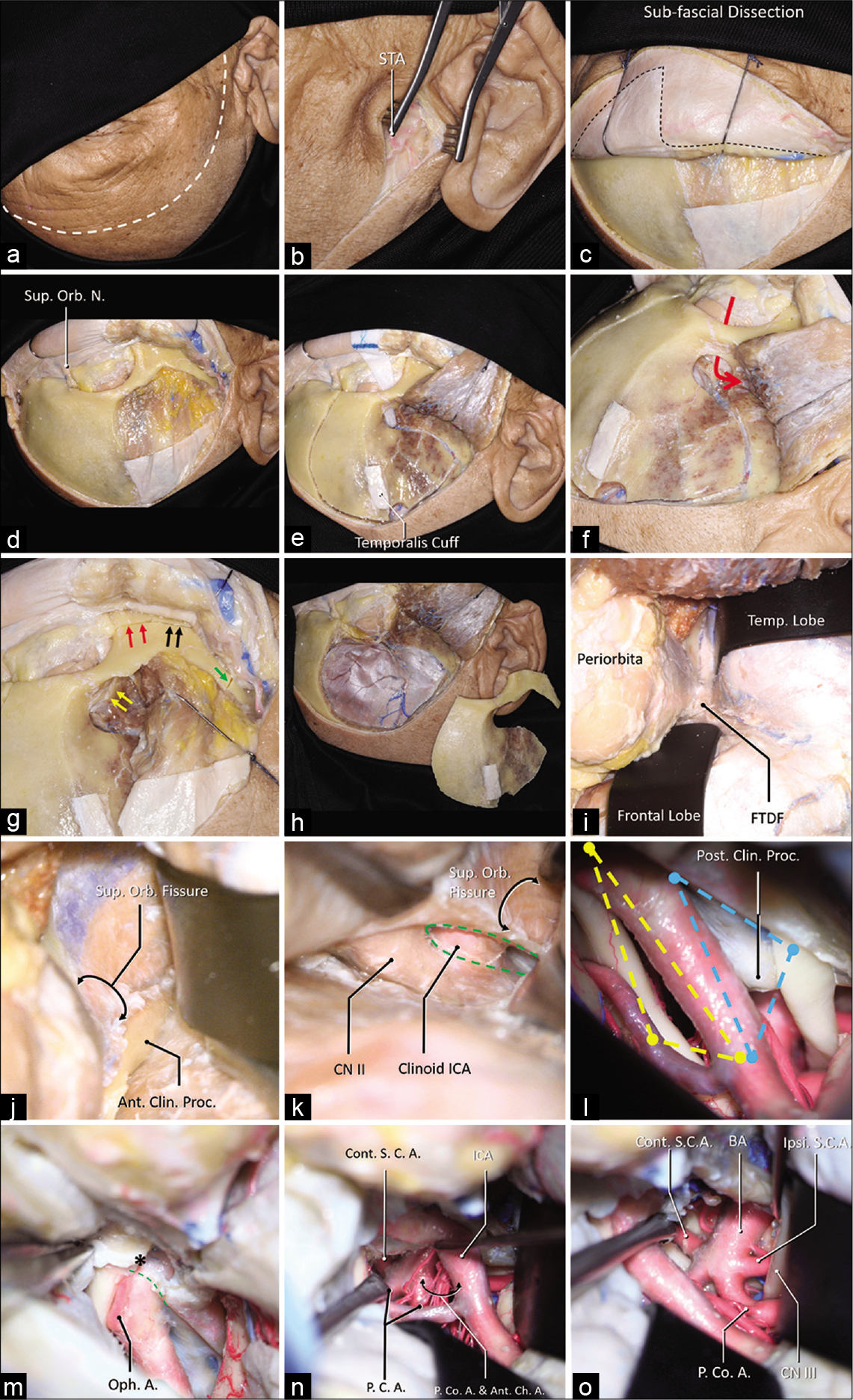
A step-by-step dissection (a) The curvilinear skin incision was demonstrated using the dashed white line, which started from 1 cm in front of the tragus and ended at the contralateral mid-pupil line. (b) The superficial temporal artery (STA) was found in the subcutaneous layer. (c) A sub-fascial dissection was performed to keep the continuation of the periosteum superior to the temporal line and the superior temporoparietal fascia inferior to the temporal line (dashed line). (d) The orbit and the zygoma were demonstrated, and the superior orbital nerve was dissected free. (e) A temporalis muscle cuff was left on the skull for reapproximation, a MacCarty keyhole at the level of the frontozygomatic suture was placed, which connects both anterior skull base and the orbit (f), the first cut was placed. (g) The MacCarty keyhole was connected to the inferior orbit fissure (yellow arrows), there were three separated cuts on the zygoma: 1, the anterior half of the zygoma was cut connecting the inferior orbital fissure (red arrows); 2, the posterior half of the zygoma was cut connecting the infratemporal fossa (black arrows); 3, the zygomatic arch was disconnected (green arrow). (h) A general view of the one-piece orbitozygomatic craniotomy. (i) After the pretemporal extradural exploration, the frontotemporal dural fold (FTDF) was demonstrated. (j) the superior orbital fissure and the nerves within were shown to be inferior to the anterior clinoid process. (k) After the anterior clinoidectomy (green dashed line), the clinoid segment of the carotid artery was demonstrated. (l) The intradural exposure showed the opitco-carotid triangle (yellow dashed line) and the carotico-oculomotor triangle (blue dashed line). (m) The distal dural ring (green dashed line) was dissected, the apex of the OCT (asterisk) was demonstrated. After the posterior clinoidectomy, the exposure to the basilar system via the OCT (n) and the COT (o) was demonstrated. Abbreviations: A., artery; Ant., anterior; Ant. Ch. A., anterior choroidal artery; BA, basilar artery; Clin., clinoid; CN, cranial nerve; Cont., contralateral; FTDF, frontotemporal dural fold; ICA, internal carotid artery; Ipsi., ipsilateral; Orb., orbital; N., nerve; Oph., ophthalmic; P.C.A., posterior cerebral artery; P. Co. A., posterior communicating artery; proc., process; S.C.A., superior cerebellar artery; STA, superficial temporal artery; Sup., superior; temp., temporal.
Quantitative measurements
In a transsylvian approach, the AIW was measured as the distance between the JAPPL on both sides.[
The OCT was measured using the following 3 points as three vertices[
The COT was measured using the following 3 points as three vertices[
A modified superior transcavernous approach was subsequently performed by opening the oculomotor triangle, and a posterior clinoidectomy was performed. The maximal width of the OCT was measured after mobilizing the ICA laterally [
Statistical analysis
In a study which investigated 100 specimens,[
As the area and the width may not have standard normal distribution, a Mann–Whitney U-test was used in the comparison. The results were presented as “mean ± standard deviation.” P < 0.05 was considered as statistically significant. The statistical analysis was performed using PASW Statistics 18.0.0 (IBM Corporation, Armonk, New York).
The Institutional Review Board approval was neither required for the anatomical cadaver dissection study and nor for the case examples description due to retrospective review and lack of identification on imaging studies.
RESULTS
The AIW was 24.3 ± 1.23 mm in the narrow group and 29.3 ± 1.59 in the wide group. No difference between the two groups was reported in terms of the area of the OCT (50.9 ± 19.22 mm vs. 63.5 ± 15.53 mm, P = 0.20), the maximal width of the OCT (6.6 ± 1.89 vs. 6.5 ± 1.38 mm, P = 1.00), or the maximal width of the COT (11.7 ± 2.06 vs. 12.2 ± 2.32 mm, P = 0.50).
The COT in the wide group was shown to have a significantly larger area compared to the COT in the narrow group (38.4 ± 12.64 vs. 58.3 ± 15.72 mm, P < 0.01).
Case examples
The AIW was measured in different clinical cases with lesions at interpeduncular fossa operated by the corresponding author as follows:
Case 1
A 56-year-old right-handed female with high blood pressure difficult to control was evaluated for unruptured 8 mm basilar apex aneurysm with high bifurcation (4 mm above posterior clinoid) [
Figure 2:
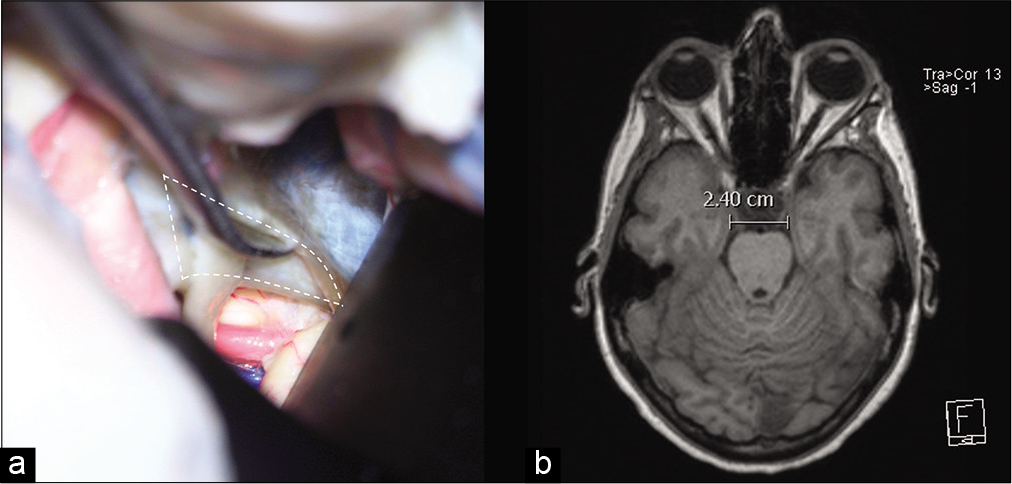
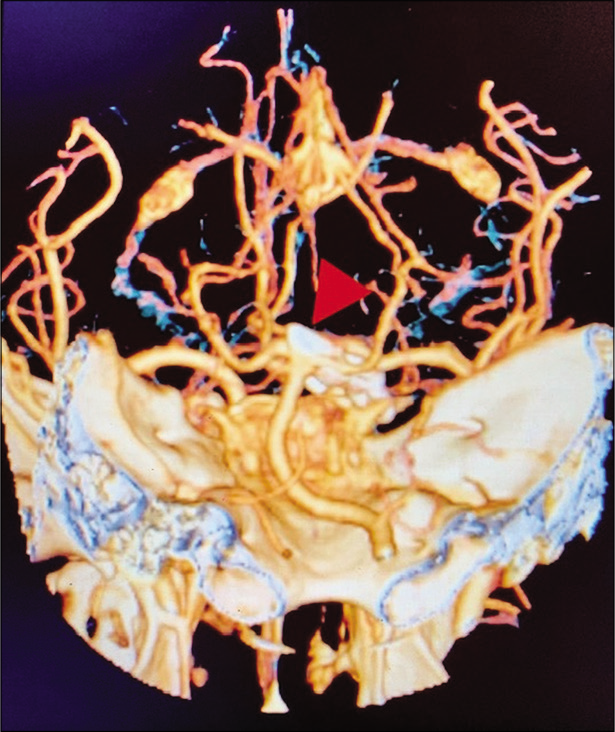
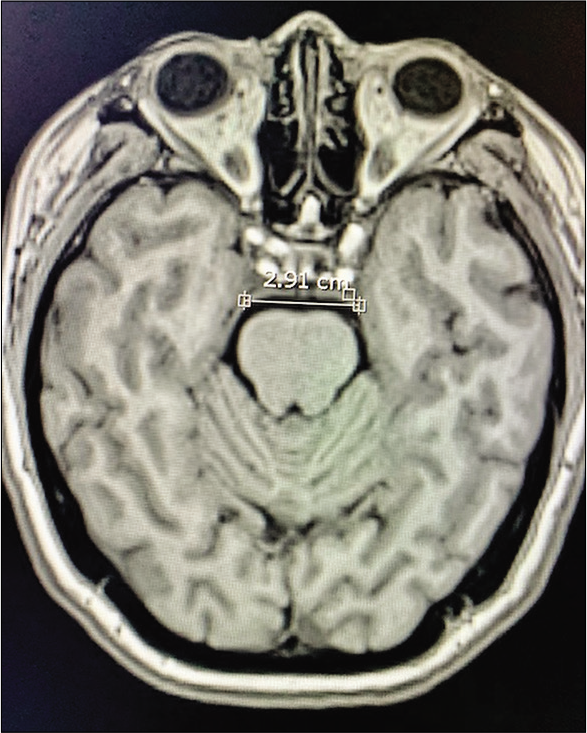
(a) The junction of the anterior petroclinoid ligament and the posterior petroclinoid ligament JAPPL was demonstrated, the anterior incisural width was measured as the distance of the JAPPL on both sides. (b) Illustration of the measurement of the AIW on an axial MRI scan. Abbreviations: JAPPL, junction of anterior and posterior petroclinoid ligaments; AIW, anterior incisural width.
Case 2
A 69-year-old right-handed female with no relevant medical history developed a sudden onset of severe headache and was found with subarachnoid hemorrhage Hunt and Hess 2, Fisher 3 with 3 mm basilar apex aneurysm rupture. The patient had significant tortuosity of vertebral arteries and, additionally, given the relatively small size of the aneurysm, our neuroendovascular team found it difficult to perform endovascular treatment [
Case 3
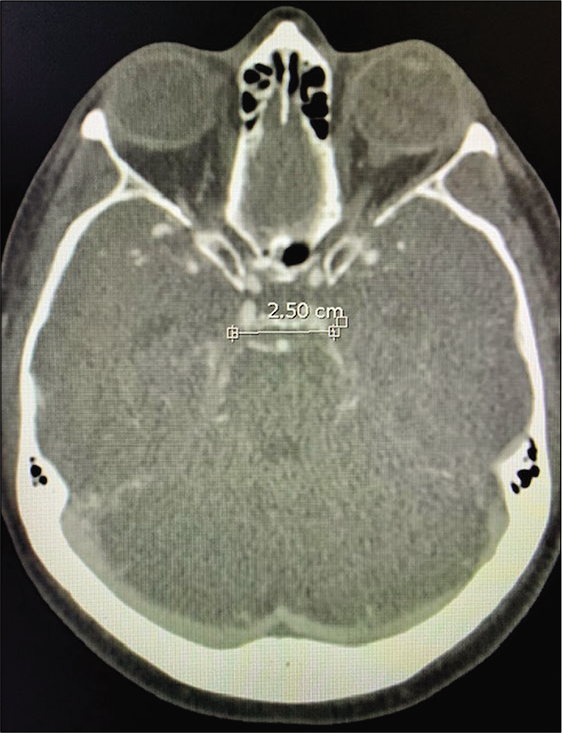
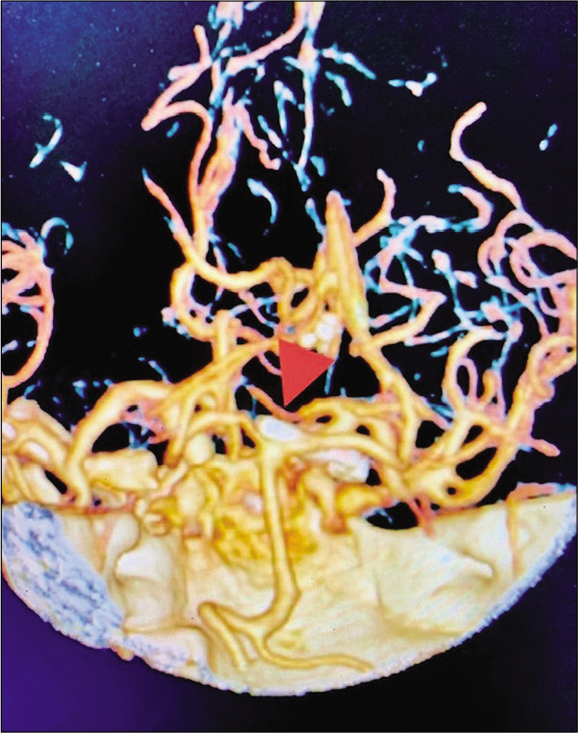
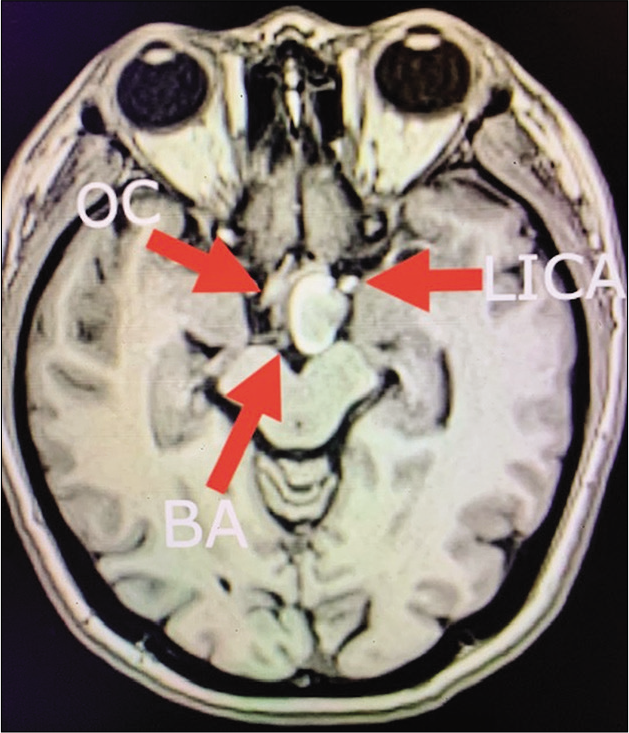
A 50-year-old female with visual field deficit – preoperative MRI showed a 2 × 1.3 × 1.3 cm (AP, transverse, and craniocaudal) extra-axial mass extending from the interpeduncular cistern to the left posterior suprasellar space, displacing the left cerebral peduncle, and the ICA laterally, and the left optic nerve and chiasm superiorly [
Figure 11:
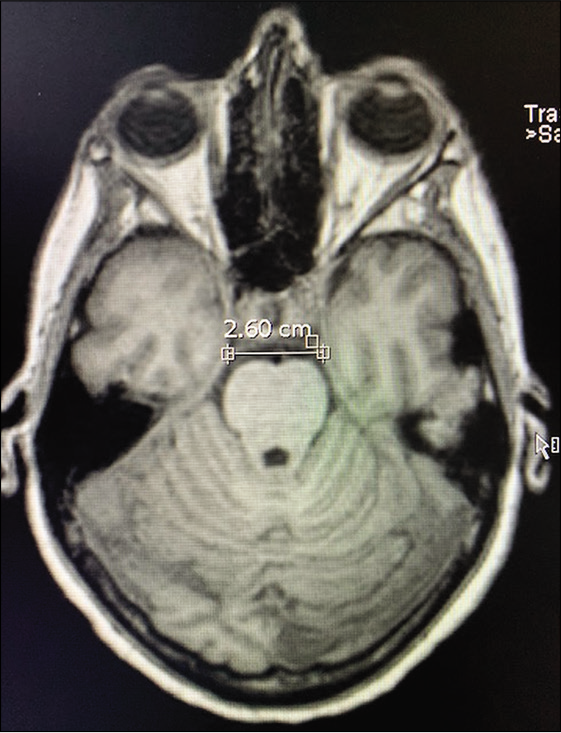
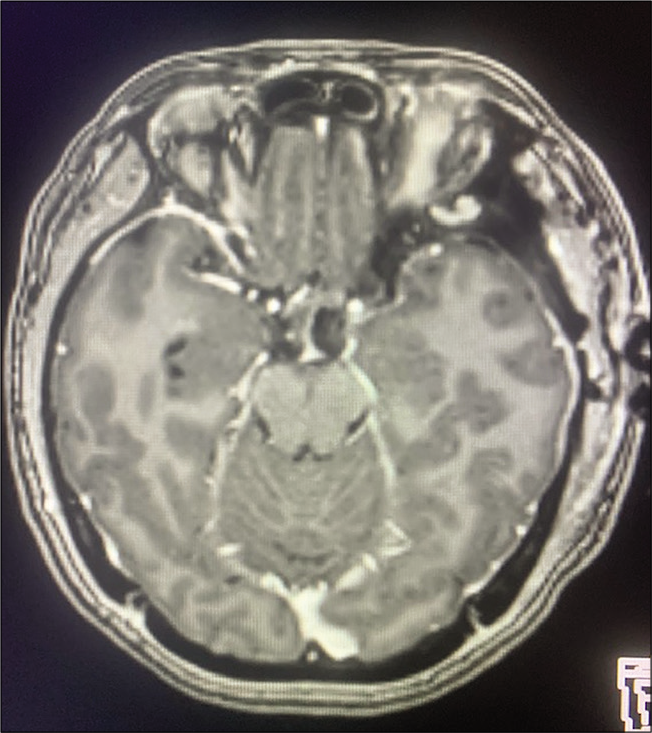
Case 3, preoperative brain MRI without contrast showing heterogenous hyperintense lesion displacing medially the optic chiasm (OC), laterally the left internal carotid artery (LICA), and located ventral and lateral to basilar artery bifurcation (BA). Abbreviations: MRI, magnetic resonance imaging; OC, optic chiasm; LICA, left internal carotid artery; BA, Basilar artery.
Figure 13:
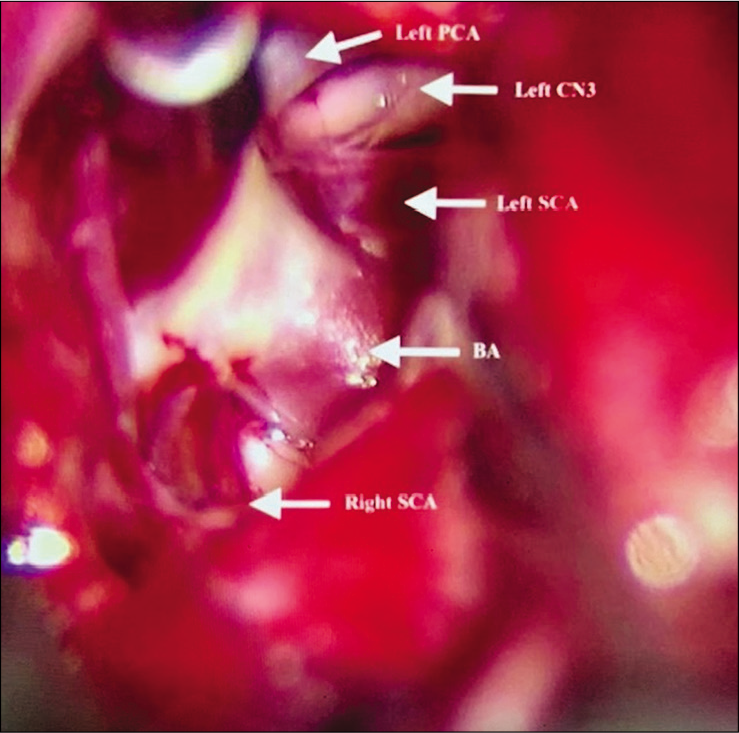
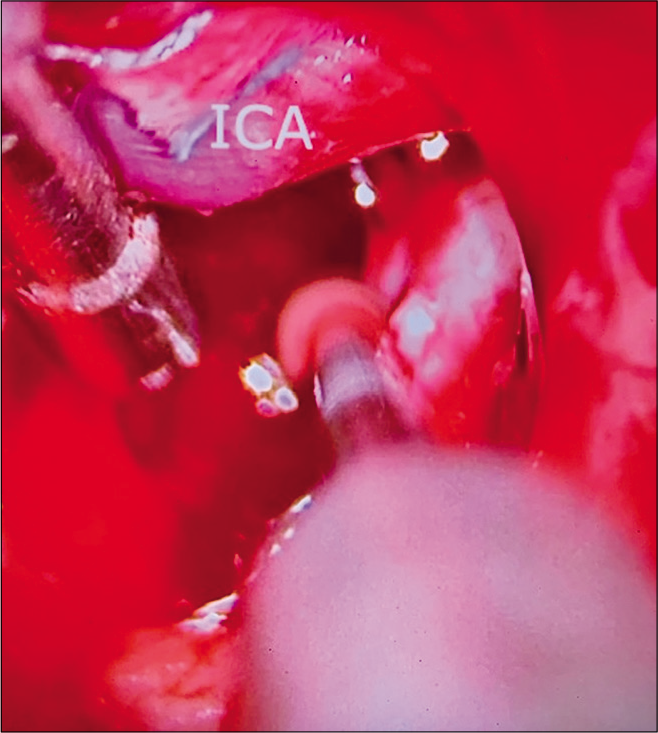
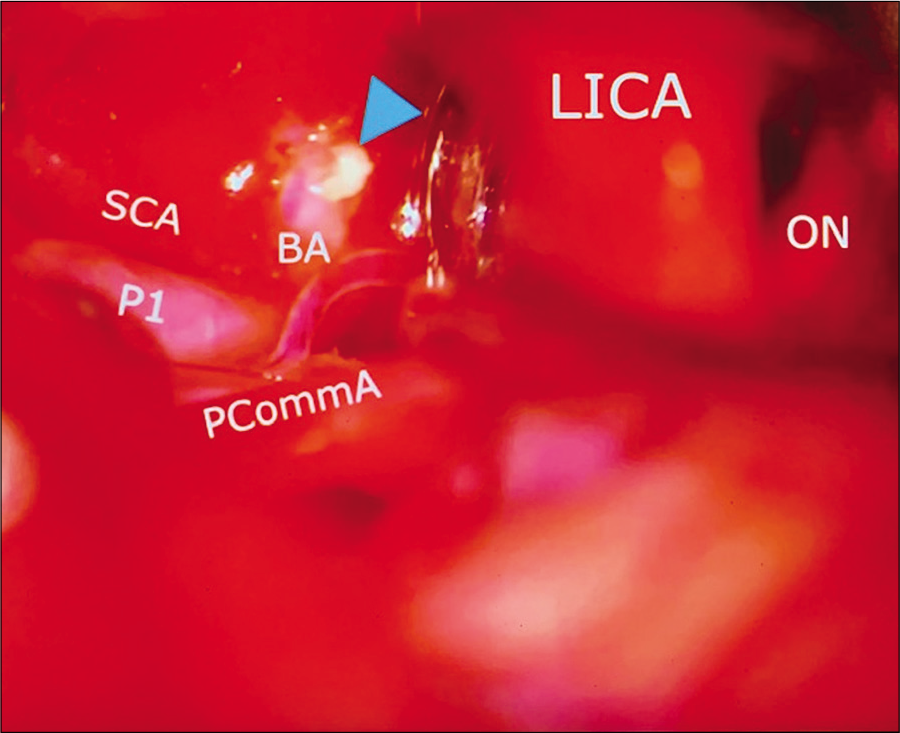
Case 3, left sided microsurgical view at COT displacing the internal carotid artery (LICA) anteriorly, and below posterior communicant artery (PCoA), exposing the basilar artery apex (BA), left posterior cerebral artery at P1 segment (P1), and left superior cerebellar artery (SCA). A small segment of residual tumor is left attached to basilar artery (arrowhead). Abbreviations: COT, carotico-oculomotor triangle; LICA, left internal carotid artery; PCoA, posterior communicant artery; BA, basilar artery apex; P1, posterior cerebral artery at P1 segment; SCA, superior cerebellar artery; ON, optic nerve.
DISCUSSION
In this manuscript, we drew the conclusion that the width of the anterior incisural space was correlated with the OCT, which can be measured preoperatively as an indicator for predicting the intradural exposure to the interpeduncular space.
Approaches and the anatomical triangles
Although there are slightly different modifications within them, lateral transcranial approaches accessing the prepontine, crural, and interpeduncular cisterns can be broadly classified as the “transsylvian approach”[
Regardless of the approach, two intradural anatomical triangles constitute major anatomical spaces through which the target region can be accessed.
Opening the OCT utilizes the space between the optic nerve and the ICA; by performing the anterior clinoidectomy and dissecting the distal and proximal dural rings, the vertex of the OCT can be extended proximally along the ICA to increase the area of the OCT.[
The COT[
Risk to take in approach selection
A transcavernous approach is optimal in terms of the exposure on the basilar artery; however, it requires pretemporal extradural exploration [
The AIW as a preoperative indicator
The distance between the JAPPL on both sides represents the width of the anterior incisural space. In our study, we reported that the AIW was correlated with the size of the COT (in the transsylvian approach), but not related with the maximum widths of the OCT and COT (in the transcavernous approach), which meant that in a transcavernous approach with the OCT and COT maximally opened, the narrow group offered similar exposure compared to the wide group. The narrow group had a smaller COT area, which is an unfavorable factor. Therefore, a transcavernous approach may be required, as in the transcavernous approach, where two groups offer similarly considerable exposure.
In preoperative settings, measuring the AIW can offer additional information to predict the size of the COT and be helpful in determining and adequately preparing if a transcavernous approach should be selected, although it is important to consider that this analysis of our case examples was retrospective.
For example, the patient on Case 1 with a high bifurcation basilar apex aneurysm had a narrow AIW of 26.0 mm, but surgical access was facilitated due to height of the basilar apex bifurcation regardless of AIW measurement; however, other factors were relevant, such as length of PCommA, no SAH, and separation between the posterior clinoid and basilar trunk. In Case 2, the estimate AIW was narrow (25.0 mm) and given basilar apex bifurcation at the level of posterior clinoid, required partial transcavernous exposure for adequate basilar apex aneurysm clipping regardless of AIW measurement. In Case 3, the wide anterior incisura of 29.1 mm, which in retrospective, we consider was a favorable factor, reflected by a wide microsurgical view for subtotal resection of tumor through the transsylvian and pretemporal approach.
Limitations of the study
The present study was performed using cadaveric specimens, the texture of which differs from that of a live tissue, in which setting the exposure can be slightly different than in real microsurgical interventions. We recognize that a limitation in our cadaveric study is the number of specimens; a larger number could have given modification of our results. More factors are involved in the approach selection, such as the height of the basilar artery bifurcation, the prominence of the posterior clinoid process, the diameter and length of the posterior communicating artery, and distance between dorsal clivus and posterior clinoid to ventral basilar artery trunk. Therefore, the approach selection should be carried out in a highly individualized fashion, with comprehensive consideration of all possible factors. Finally, a brain MRI is more favorable than CTA to clearly identify AIW. However, in practical scenarios, it is uncommon for patients with aneurysm lesions to undergo an MRI scan, which is also financially disadvantageous.
CONCLUSION
The AIW is a potential preoperative indicator of the size of the COT. An AIW <26.6 mm is considered “narrow,” which is related to a small area of the COT. We consider this an unfavorable factor for a transsylvian approach in lesions located at interpeduncular cistern, and a transcavernous approach can be considered under this circumstance. The AIW is only one element that we suggest can be taken into account during preoperative planning among multiple other elements for surgery in these areas. A larger series with measurement of AIW’s can help to define its real use as a predictive tool for favorable microsurgical view and approach selection.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Adler DE, Milhorat TH. The tentorial notch: Anatomical variation, morphometric analysis, and classification in 100 human autopsy cases. J Neurosurg. 2002. 96: 1103-12
2. Aziz KA, Froelich SC, Cohen PL, Sanan A, Keller JT, van Loveren HR. The one-piece orbitozygomatic approach: The MacCarty burr hole and the inferior orbital fissure as keys to technique and application. Acta Neurochir (Wien). 2002. 144: 15-24
3. Basma J, Ryttlefors M, Latini F, Pravdenkova S, Krisht A. Mobilization of the transcavernous oculomotor nerve during basilar aneurysm surgery: Biomechanical bases for better outcome. Neurosurgery. 2014. 10: 106-15
4. Bendok BR, Getch CC, Parkinson R, O’Shaughnessy BA, Batjer HH. Extended lateral transsylvian approach for basilar bifurcation aneurysms. Neurosurgery. 2004. 55: 174-8
5. Coscarella E, Başkaya MK, Morcos JJ. An alternative extradural exposure to the anterior clinoid process: The superior orbital fissure as a surgical corridor. Neurosurgery. 2003. 53: 162-7
6. Dolenc VV, Škrap M, Šušteršič J, Skrbec M, Morina A. A transcavernous-transsellar approach to the basilar tip aneurysms. Br J Neurosurg. 1987. 1: 251-9
7. Evans JJ, Hwang YS, Lee JH. Pre-versus post-anterior clinoidectomy measurements of the optic nerve, internal carotid artery, and opticocarotid triangle: A cadaveric morphometric study. Neurosurgery. 2000. 46: 1018-23
8. Everton KL, Rassner UA, Osborn AG, Harnsberger HR. The oculomotor cistern: Anatomy and high-resolution imaging. AJNR Am J Neuroradiol. 2008. 29: 1344-8
9. Figueiredo EG, Zabramski JM, Deshmukh P, Crawford NR, Preul MC, Spetzler RF. Anatomical and quantitative description of the transcavernous approach to interpeduncular and prepontine cisterns. J Neurosurg. 2006. 104: 957-64
10. Froelich SC, Aziz KM, Levine NB, Theodosopoulos PV, van Loveren HR, Keller JT. Refinement of the extradural anterior clinoidectomy: Surgical anatomy of the orbitotemporal periosteal fold. Neurosurgery. 2007. 61: 179-85
11. Gibo H, Lenkey C, Rhoton AL. Microsurgical anatomy of the supraclinoid portion of the internal carotid artery. J Neurosurg. 1981. 55: 560-74
12. Hsu FP, Clatterbuck RE, Spetzler RF. Orbitozygomatic approach to basilar apex aneurysms. Neurosurgery. 2005. 56: 172-7
13. Isolan GR, Krayenbühl N, de Oliveira E, Al-Mefty O. Microsurgical anatomy of the cavernous sinus: Measurements of the triangles in and around it. Skull Base. 2007. 17: 357-67
14. Krisht AF, Kadri PA. Surgical clipping of complex basilar apex aneurysms: A strategy for successful outcome using the pretemporal transzygomatic transcavernous approach. Neurosurgery. 2005. 56: 261-73
15. Lemole GM, Henn JS, Zabramski JM, Spetzler RF. Modifications to the orbitozygomatic approach. Technical note. J Neurosurg. 2003. 99: 924-30
16. Sade B, Kweon CY, Evans JJ, Lee JH. Enhanced exposure of carotico-oculomotor triangle following extradural anterior clinoidectomy: A comparative anatomical study. Skull Base. 2005. 15: 157-61
17. Tayebi MA, Borba LM, Zhao X, Lawton MT, Preul MC. Transcavernous approach to the upper basilar and retroclival area-cadaveric surgical simulation video: 2-Dimensional operative video. Oper Neurosurg (Hagerstown). 2019. 17: E251-
18. Zabramski JM, Kiriş T, Sankhla SK, Cabiol J, Spetzler RF. Orbitozygomatic craniotomy. Technical note. J Neurosurg. 1998. 89: 336-41