- Department of Neurosurgery, University of Liège, Liège, Belgium.
- Department of Medical Imaging, University of Liège, Liège, Belgium.
- Department of Human Systematic Anatomy University of Liège, Liège, Belgium.
- Department of Neuroanatomy, University of Liège, Liège, Belgium.
Correspondence Address:
Felix Scholtes, Departments of Neurosurgery and Neuroanatomy, University of Liège, Liège, Belgium.
DOI:10.25259/SNI_333_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Julie Lebeau1, Martin Moïse2, Pierre Bonnet3, Didier Herman Martin1, Bernard Otto2, Felix Scholtes1,4. The dural vascular plexus in subdural hematoma: Illustration through a case of dural arteriovenous fistula. 20-May-2022;13:212
How to cite this URL: Julie Lebeau1, Martin Moïse2, Pierre Bonnet3, Didier Herman Martin1, Bernard Otto2, Felix Scholtes1,4. The dural vascular plexus in subdural hematoma: Illustration through a case of dural arteriovenous fistula. 20-May-2022;13:212. Available from: https://surgicalneurologyint.com/surgicalint-articles/the-dural-vascular-plexus-in-subdural-hematoma-illustration-through-a-case-of-dural-arteriovenous-fistula/
Abstract
Background: The initiation of chronic subdural hematoma (cSDH) is traditionally explained by rupture of bridging veins. Recent descriptions of the embryology and anatomy of the meninges and their vascularization, however, point to the dural vascular plexus (DVP) as a plausible origin of cSDH. This dural plexus is supplied by meningeal arteries. Their endovascular occlusion is efficient in cSDH treatment. Dural arteriovenous fistulae (dAVF) may also present with subdural hematoma.
Case Description: A 65-year-old female patient presented with parietal parasagittal dAVF and bilateral cSDH requiring surgical disconnection followed by complete clinical and imaging resolution of dAVF and cSDH.
Conclusion: In common cSDH, pressure in the DVP may be normal and subdural bleeding may occur due to mechanical traction on the DVP. In the setting of dAVF, it may be the increase in pressure due to the fistula, within the DVP, that causes subdural hematoma. The DVP, supplied by meningeal arteries, thus not only allows for convergent pathophysiological explanation of subdural bleeding in both cSDH and dAVF but may also be the actual target of the emergent endovascular treatment of cSDH trough meningeal artery embolization.
Keywords: Bridging veins, Dural arteriovenous fistula, Dural vascular plexus, Meningeal artery embolization, Subdural hematoma
INTRODUCTION
Intracranial dural arteriovenous fistulae (dAVF) are shunts between meningeal arteries and cerebral veins which result in cerebral venous hypertension. Most become symptomatic through parenchymal hemorrhage or nonhemorrhagic neurological deficits associated with edema or ischemia.[
CASE PRESENTATION
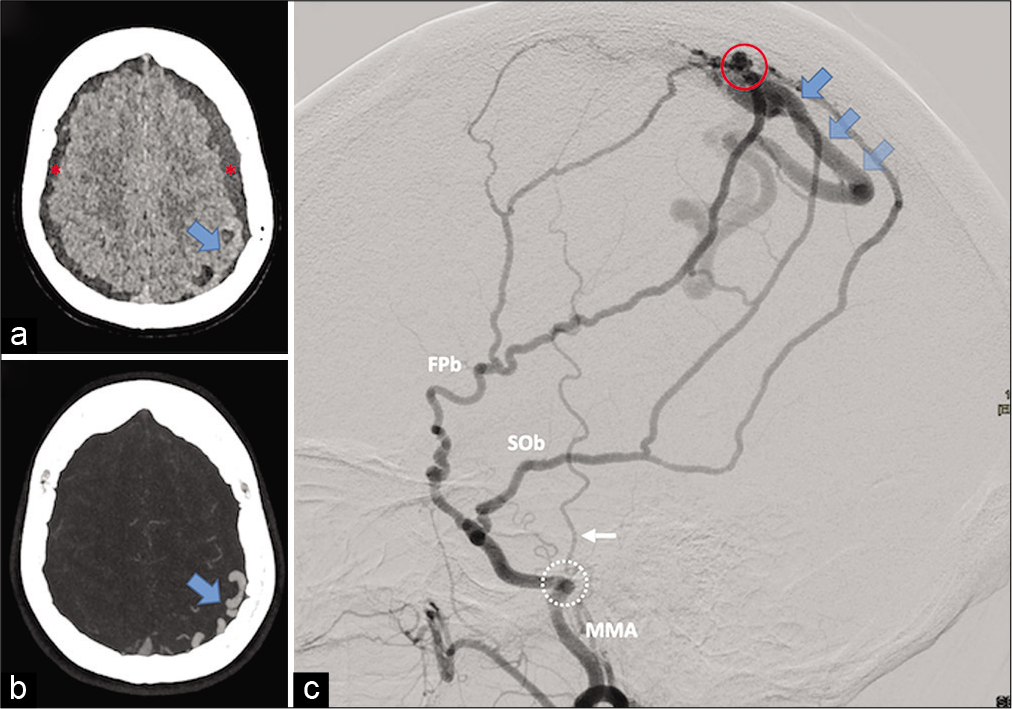
A 65-year-old woman, without known head trauma, craniotomy, or dural sinus thrombosis, presented with a 2-month history of headache and neck pain which had progressively worsened and were associated with nausea over the 5 preceding days. There was no neurological deficit at examination. Cranial computed tomography (CT) and magnetic resonance imaging showed bilateral cSDH, as well as abnormal left parasagittal vascular structures. Digital subtraction angiography confirmed these to be a dAVF, fed bilaterally by parieto-occipital branches of the middle meningeal artery (MMA) draining directly into a unique dilated left parietal cortical vein without venous ectasia and secondary drainage within the superior longitudinal sinus (Cognard Grade 3) [
Figure 1:
Preoperative imaging. (a and b) Head computed tomography scan. A (noncontrast acquisition): bilateral cSDH (red asterixis) and left extra-axial structures suspected to be abnormal vessels (blue arrow). (b) (Maximum Intensity Projection [MIP] reconstruction from CT angiography): the structures are identified as dilated cortical veins (blue arrow). C (left middle meningeal artery [MMA] digital subtracted angiography [DSA], lateral view): dAVF depending on frontoparietal and squamo-occipital MMA branches (respectively, FPb and SOb). The shunt (red circle) is directly on a tortuous left parietal cortical vein (blue arrows) with secondary drainage within the superior longitudinal sinus (Cognard Type III dAVF). The foramen spinosum (white dotted circle) and the temporal artery (white arrow) are depicted as anatomical landmarks.
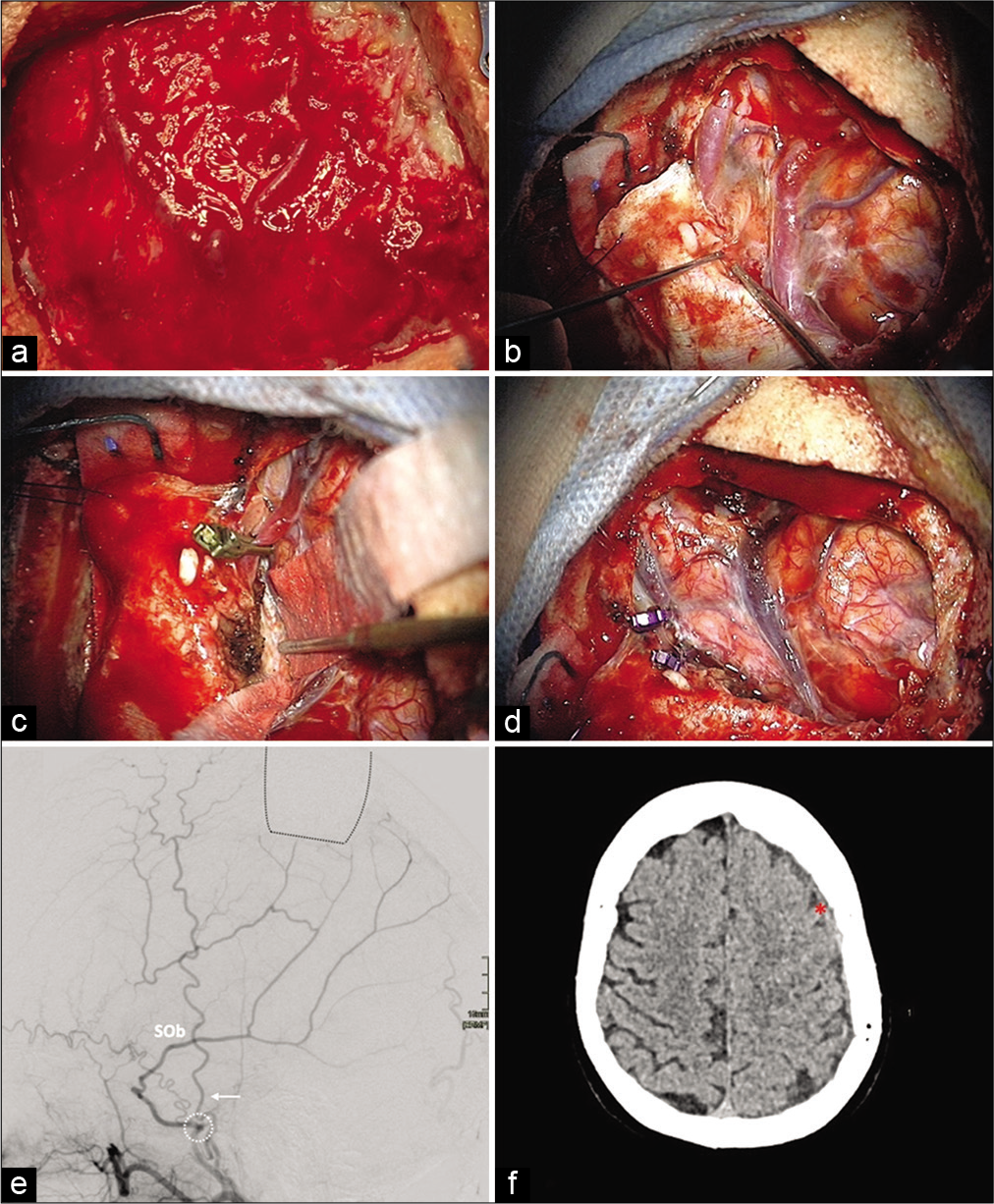
An endovascular attempt to disconnect the fistula failed because of difficulties navigating within the draining vein and failure to reach the shunt point with injected liquid embolization agent from the arterial side. Postembolization CT showed recent hemorrhage within the left subdural collection in the absence of clinical deterioration. The fistula was approached through a left parietal craniotomy and located within a group of parasagittal calcifications. After temporary clipping, demonstrating dearterialization of the draining vein, the fistula was definitively clipped and sectioned. The deflated draining vein’s color changed, as expected, from bright red to dark purple [
Figure 2:
Intraoperative images of different stages of the operation (A-D) and postoperative imaging. (a) Turgescent dural vessels. (b) Intradural view of turgescent veins located within a group of parasagittal calcifications. (c) Transient clipping of the posterior vein: deflation and color change indicating the dearterialization of the draining veins. (d) Clipping and division of the coagulated vein with macroscopic normalization of the venous system. (e) (Lateral view of postoperative MMA DSA, 18 days): no residual early venous opacification. Now that the shunt has been disconnected, the occipital component of the SOb is more clearly observed. The proximal part of the FPb had been occluded during the embolization attempt. The localization of the craniotomy flap (back dotted lines), the foramen spinosum (white dotted circle), and the temporal artery (white arrow) are depicted as anatomical landmarks. (f) (Noncontrast CT, 34 days): regression of the left SDH (red asterisk).
DISCUSSION
Subdural hematoma in dAVF has traditionally been attributed to ruptured arterialized bridging veins.[
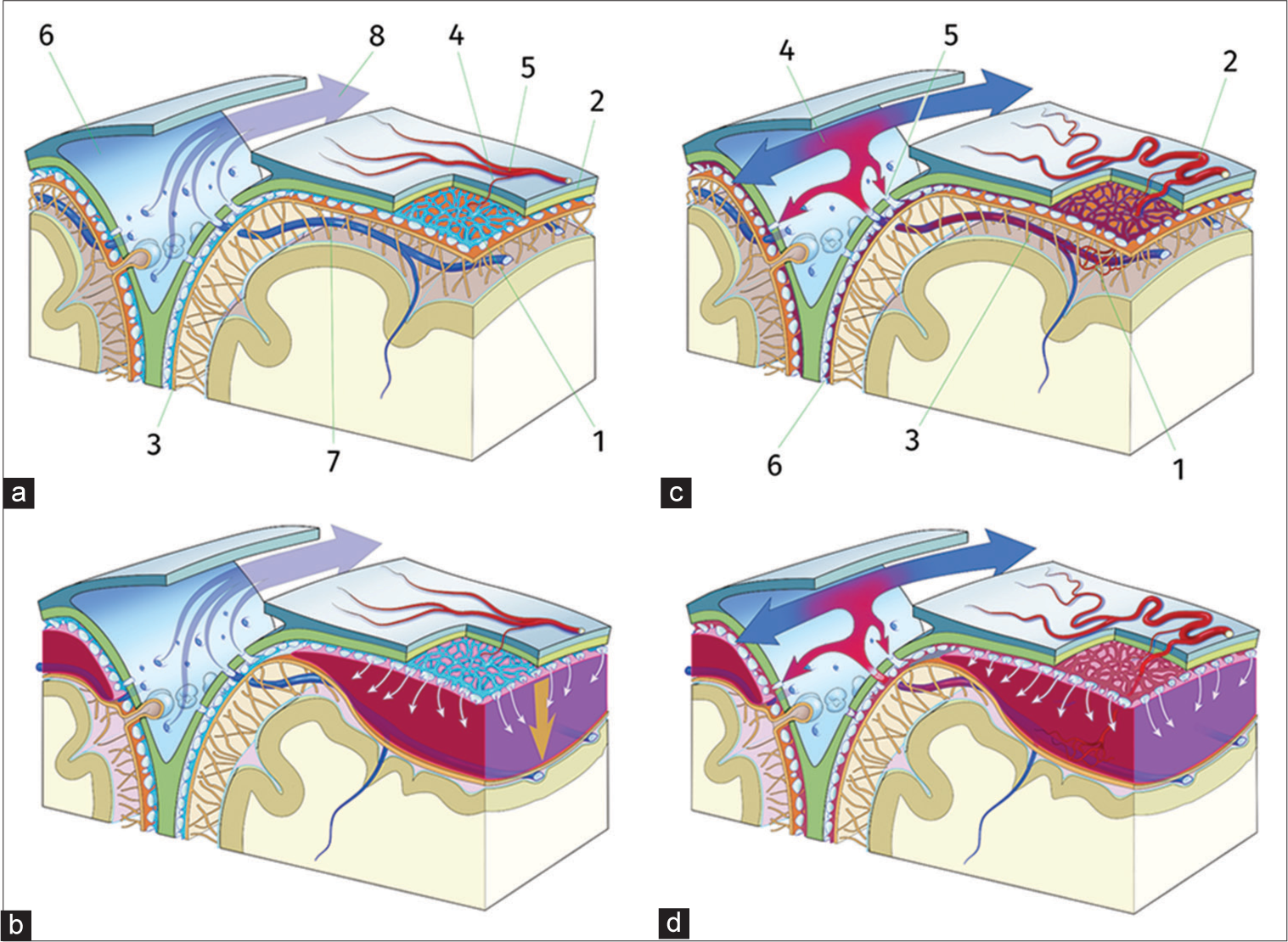
Figure 3:
(a) The DVP (1) is located in the dural border cells layer between the dura mater (2) and the arachnoid membrane (3). It is fed by branches (4) of the meningeal artery (5) and it drains directly into the superior sagittal sinus (6) and its lateral pouches, independently from cortical bridging veins (7), with unidirectional flow (8). (b) Common cCSH: traction (arrow) on the dural border cells layer, for example, with minor head trauma and subsequent blood accumulation, leading to hemorrhage from the inner dural plexus (white arrows) creating cSDH by disruption of the border cell layer joining arachnoid membrane and dura mater. (c) Cognard Type III dAVF (1) between the tortuous meningeal artery (2) and a cortical vein. Blood flow is arterialized (3), leading to local pressure increase in the SSS (4) and creating an high pressure reflux (5) into the DVP (6) and abnormal blood flow in the SSS. (d) Increase in DVP pressure leads to rupture and, thus, subdural bleeding.
Common cSDH mainly occurs in elderly patients with relatively atrophic brains. Bleeding may be initiated by traction on the inner dural border cell plexus in these aging meninges [
In the setting of dAVF, on the other hand, subdural bleeding is attributed to venous hypertension resulting from the arteriovenous shunt. Since the DVP drains directly into the cerebral sinus, separately from the sites of entry of the bridging veins,[
Therefore, increased pressure within the DVP provides a plausible explanation for the occurrence of subdural hematoma in the setting of dAVF, including the case we describe, which is only the second case of bilateral SDH associated with dAVF reported in the literature to the best of our knowledge.[
CONCLUSION
The DVP is a plausible origin of subdural hematoma. The present case of bilateral SDH due to dAVF can satisfactorily be explained by sinus hypertension that increased pressure in the DVP. Changes in the pressure regime of the DVP may also play a role in the efficacy of the endovascular meningeal arterial devascularization in common cSDH.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Bhogal P, Yeo LL, Söderman M. Dural arteriovenous fistula presenting as tentorial subdural hemorrhage: Case report and review of the literature. Clin Neuroradiol. 2019. 29: 555-61
2. Cheshire EC, Malcomson RD, Joseph S, Adnan A, Adlam D, Rutty GN. Post-mortem imaging of the infant and perinatal Dura mater and superior sagittal sinus using optical coherence tomography. Int J Legal Med. 2017. 131: 1377-83
3. Choi JH, Jo KI, Kim KH, Jeon P, Yeon JY, Kim JS. Early rebleeding of intracranial dural arteriovenous fistulas after an intracranial hemorrhage. Acta Neurochir. 2017. 159: 1479-87
4. Cognard C, Casasco A, Toevi M, Houdart E, Chiras J, Merland JJ. Dural arteriovenous fistulas as a cause of intracranial hypertension due to impairment of cranial venous outflow. J Neurol Neurosurg Psychiatry. 1998. 65: 308-16
5. Edlmann E, Giorgi-Coll S, Whitfield PC, Carpenter KL, Hutchinson PJ. Pathophysiology of chronic subdural haematoma: Inflammation, angiogenesis and implications for pharmacotherapy. J Neuroinflammation. 2017. 14: 108
6. Elhammady MS, Ambekar S, Heros RC. Epidemiology, clinical presentation, diagnostic evaluation, and prognosis of cerebral dural arteriovenous fistulas, in Handb Clin Neurol. 2017. 143: 99-105
7. Fiorella D, Arthur AS. Middle meningeal artery embolization for the management of chronic subdural hematoma. J Neurointervent Surg. 2019. 11: 912-5
8. Gross BA, Albuquerque FC, Moon K, McDougall CG. Evolution of treatment and a detailed analysis of occlusion, recurrence, and clinical outcomes in an endovascular library of 260 dural arteriovenous fistulas. J Neurosurg. 2016. 126: 1884-93
9. Han H, Tao W, Zhang M. The dural entrance of cerebral bridging veins into the superior sagittal sinus: An anatomical comparison between cadavers and digital subtraction angiography. Neuroradiology. 2007. 49: 169-75
10. Kohyama S, Ishihara S, Yamane F, Kanazawa R, Ishihara H. Dural arteriovenous fistula presenting as an acute subdural hemorrhage that subsequently progressed to a chronic subdural hemorrhage: Case report. Minim Invasive Neurosurg. 2009. 52: 36-8
11. Kwon BJ, Han MH, Kang HS, Chang KH. MR imaging findings of intracranial dural arteriovenous fistulas: Relations with venous drainage patterns. AJNR Am J Neuroradiol. 2005. 26: 2500-7
12. Lamas E, Lobato RD, Esparza J, Escudero L. Dural posterior fossa AVM producing raised sagittal sinus pressure: Case report. J Neurosurg. 1977. 46: 804-10
13. Mack J, Squier W, Eastman JT. Anatomy and development of the meninges: Implications for subdural collections and CSF circulation. Pediatr Radiol. 2009. 39: 200-10
14. Masaki K, Toshihiro Y, Katsuhiko T, Yasunori N, Yoshihiko F, Hisatsugu Y. Chronic subdural hematoma associated with middle meningeal arteriovenous fistula treated by a combination of embolization and burr hole drainage. Surg Neurol. 1994. 43: 316-9
15. Reynolds MR, Lanzino G, Zipfel GJ. Intracranial dural arteriovenous fistulae. Stroke. 2017. 48: 1424-31
16. Rowbotham GF, Little E. New concepts on the aetiology and vascularization of meningiomata; the mechanisms of migraine; the chemical processes of the cerebrospinal fluid; and the formation of collections of blood or fluid in the subdural space. Br J Surg. 2005. 52: 21-4
17. Shapiro M, Walker M, Carroll KT, Levitt MR, Raz E, Nossek E. Neuroanatomy of cranial dural vessels: implications for subdural hematoma embolization. J NeuroIntervent Surg. 2021. 13: 471-7
18. Squier W, Mack J. The neuropathology of infant subdural haemorrhage. Forensic Sci Int. 2009. 187: 6-13
19. Suyama Y, Wakabayashi S, Kajikawa H, Nakahara I. Dural arteriovenous fistula presenting with acute subdural haematoma showing impending cerebral herniation. BMJ Case Rep. 2018. 2018: bcr2017223177
20. Yamauchi K, Takenaka S, Iida T, Sakai H. A case of spontaneous acute subdural hemorrhage caused by a dural arteriovenous fistula on the convexity without cortical venous reflux. Case Rep Neurol. 2019. 11: 312-8