- Department of Neurosurgery, School of Medicine, Kurume University, Kurume City, Fukuoka, Japan.
- Department of Radiology, School of Medicine, Kurume University, Kurume City, Fukuoka, Japan.
Correspondence Address:
Takahisa Nonaka, Department of Neurosurgery, School of Medicine, Kurume University, Kurume City, Fukuoka, Japan.
DOI:10.25259/SNI_808_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Takahisa Nonaka1, Kiyohiko Sakata1, Toshi Abe2, Gohsuke Hattori1, Kimihiko Orito1, Naohisa Miyagi1, Takashi Tokutomi1, Motohiro Morioka1. The eagle jugular syndrome as the cause of delayed intracranial hemorrhage after microvascular decompression for hemifacial spasm: A case report. 30-Nov-2021;12:584
How to cite this URL: Takahisa Nonaka1, Kiyohiko Sakata1, Toshi Abe2, Gohsuke Hattori1, Kimihiko Orito1, Naohisa Miyagi1, Takashi Tokutomi1, Motohiro Morioka1. The eagle jugular syndrome as the cause of delayed intracranial hemorrhage after microvascular decompression for hemifacial spasm: A case report. 30-Nov-2021;12:584. Available from: https://surgicalneurologyint.com/surgicalint-articles/11257/
Abstract
Background: Eagle syndrome is a rare disorder whereby an elongated styloid process (ESP) causes not only some otolaryngological symptoms, but also cerebrovascular events caused by compression of the carotid artery. In recent years a syndrome, denominated as Eagle jugular syndrome, involving internal jugular vein (IJV) compression caused by an ESP has been proposed as a variation of Eagle syndrome. Clinical impact of the Eagle jugular syndrome on neurosurgical procedures has not been reported yet.
Case Description: We present a case of a 68-year-old woman who underwent microvascular decompression for hemifacial spasm of the left side and developed delayed intracranial hemorrhage on postoperative day 3. We also demonstrate that this patient developed ipsilateral IJV stenosis between an ESP and the muscle bundle of the rectus capitis lateralis with antero-flexion neck position, which would induce venous congestion in addition to surgical disruption of emissary vein.
Conclusion: This case is the first report demonstrating the association of an ESP with postoperative delayed intracranial hemorrhage. Our report elucidates the importance of the awareness among neurosurgeons of considering the ESP as an important bony anomaly, especially when planning for posterior fossa surgery.
Keywords: Delayed intracranial hemorrhage, Eagle jugular syndrome, Elongated styloid process, Hemifacial spasm, Internal jugular vein stenosis, Microvascular decompression
BACKGROUND
The styloid process is a slender bony projection arising from the lower surface of the petrous portion of the temporal bone. In 1937, Eagle described two cases in which cervicofacial pain, dysphagia, and otalgia were caused by an elongated styloid process (ESP), which is well known as the Eagle syndrome.[
Microvascular decompression (MVD) is a type of comparatively safe and effective functional surgery for hemifacial spasm (HFS) that has a cure rate of approximately 85–90% with < 10% operative morbidity.[
CASE DESCRIPTION
A 68-year-old woman who presented with a 5-year history of typical left HFS was admitted to our hospital for neurosurgical treatment. Magnetic resonance (MR) imaging revealed the presence of neurovascular compression at the root exit zone (REZ) of the left facial nerve by the left anterior inferior cerebellar artery [
Figure 1:
Preoperative images on heavily T2-weighted magnetic resonance (MR) image (a) and source image of MR angiography (b) showing neurovascular compression at the root exit zone (REZ) of the left facial nerve by the anterior inferior cerebellar artery. Intraoperative photographs showing (c) the REZ of the affected nerve (Asterisk) was readily relieved by the offending artery (Black arrow) with minimum manipulation. Postoperative day 3, (d) head computed tomography image showing a subdural hematoma in the left cerebellopontine angle (CPA). Outstanding high-density lesion at the REZ is surgical prosthesis (Black arrow). T2-weighted MR images (e, f) showing a subdural hematoma in the left CPA and a cerebellar hemorrhage with perifocal edema that could indicate venous infarction.
Postoperative computed tomography (CT) scan on the next morning after surgery revealed no abnormal findings. Except for complain of headache, the postoperative course appeared uneventful. However, on the 3rd postoperative day, her conscious level deteriorated while asleep. Whereas she was resting sideways due to the pain of the wound for a while after the operation, her head position had been raised with a pillow at that time. CT scan revealed a subdural hematoma in the left cerebellopontine angle (CPA) with hydrocephalus [
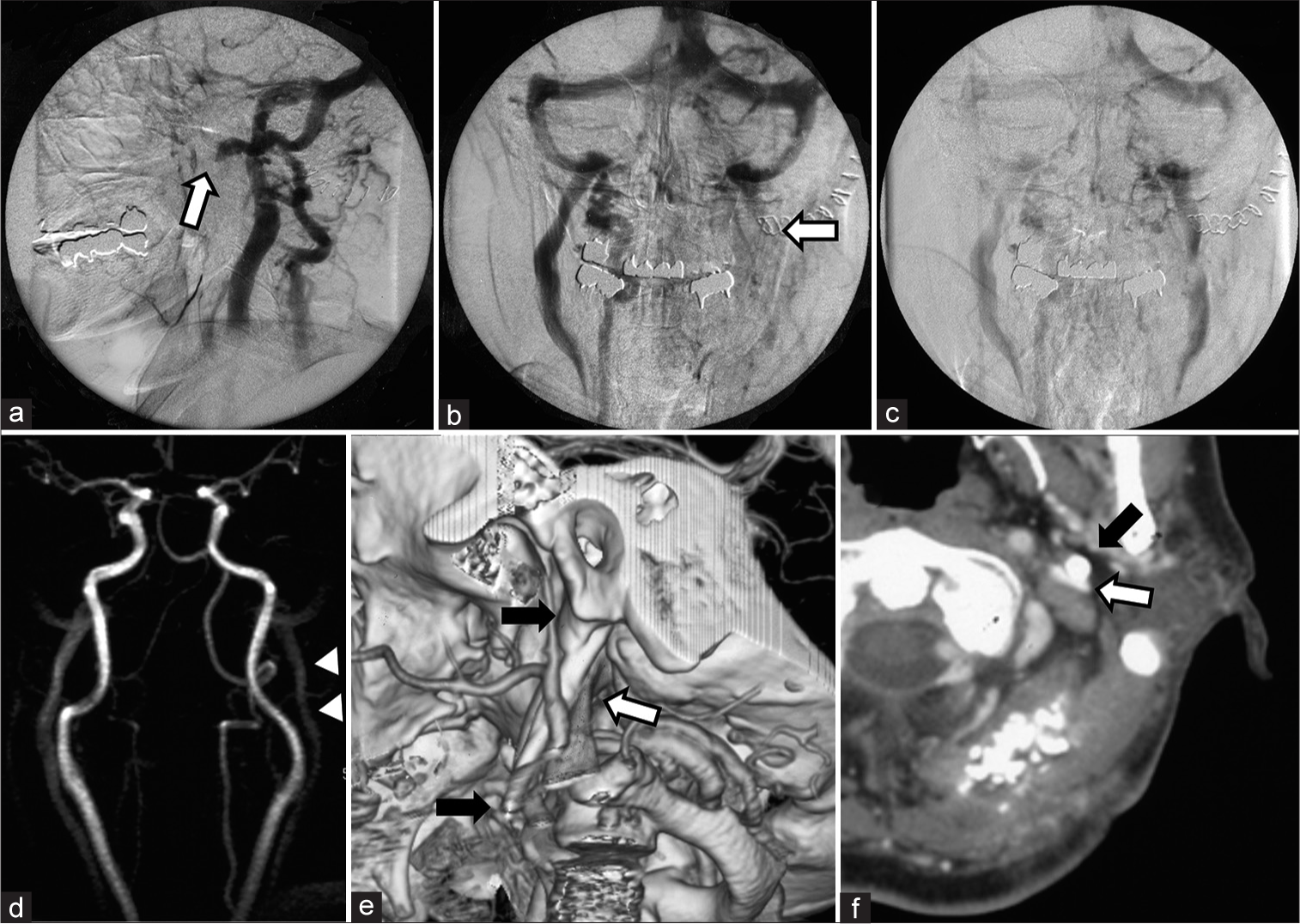
Figure 2:
Investigations for the causality of delayed intracranial hemorrhage revealed (a, b) interruption of the ipsilateral internal jugular vein (IJV) at the level of the C1 (White arrow) on angiography. (c) The venous outflow was congested but not occluded, which revealed on further delayed phase. (d) Preoperative MR angiogram showed no stenosis at this portion of the IJV (Arrow head). (e) The three-dimensional computed tomography (3D-CT) venogram showing an elongated styloid process (ESP) that was 48 mm in length (Black arrow) and compressed the IJV at the level of the third segment of the vertebral artery (White arrow). (f) The former axial images of the 3D-CT venogram also showed that the IJV (White arrow) was pinched between the ESP (Black arrow) and the rectus capitis lateralis. It is notable that the transverse process of C1 vertebra did not contribute to the pinching directly in this case.
Figure 3:
Magnetic resonance (MR) venography showing alteration of the signal flow on the bilateral transverse-sigmoid sinus. (a) MR venography with posterior-extension of the neck position showing high-signal flow on the left transverse-sigmoid sinus. (b) MR venography with antero-flexion of the neck position showing significant reduction of the signal flow on the affected side (a and b; White arrow).
DISCUSSION
Anatomically, the styloid process projects inferiorly and anteriorly into the parapharyngeal space where it is in close proximity to numerous structures. The retrostyloid compartment contains the IJV, the ICA, the sympathetic chain, and the glossopharyngeal, vagus, accessory, and hypoglossal nerves. The prestyloid compartment contains the internal maxillary artery, the lingual and auriculotemporal nerves, and is related to the tonsillar fossa inferiorly.[
Eagle syndrome presents various symptoms due to the mechanical compression of the surrounding anatomical structures by the ESP, which is occasionally triggered by neck mobility including flexion/extension and contralateral rotation.[
Delayed intracranial hemorrhage after MVD has been rarely reported (with an incidence of 0–0.4%) as one of postoperative fatal complications.[
While this complication was unpredictable during the intraoperative procedure and, unfortunately, preoperative routine examinations, we speculate that the causes of delayed hemorrhage may be both maintaining a specific neck position and the disconnection of these compensable collateral venous channels such as the mastoid emissary vein, whose disruption is often unavoidable during lateral suboccipital craniotomy.[
In addition, the structure opposite of the ESP to pinch the IJV is the transverse process of the C1 vertebra in most of the studies;[
CONCLUSION
Postoperative delayed intracranial hemorrhage after MVD is an unexpected and fatal complication. Whereas Eagle jugular syndrome is a venous variant of the Eagle syndrome, neurosurgeons should regard the ESP as an important bony structure, which has the potential to induce venous congestion by compressing the IJV, especially when planning for posterior fossa surgery.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We thank the nursing and anesthesiology teams for the perioperative management of the patient who underwent neurological surgery. We would like to thank Editage (www.editage.com) for English language editing.
References
1. Bafaqeeh SA. Eagle syndrome: Classic and carotid artery types. J Otolaryngol. 2000. 29: 88-94
2. Bai C, Wang Z, Guan J, Jin K, Ding Y, Ji X. Clinical characteristics and neuroimaging findings in eagle syndrome induced internal jugular vein stenosis. Ann Transl Med. 2020. 8: 97
3. Baldino G, Di Girolamo C, De Blasis G, Gori A. Eagle syndrome and internal carotid artery dissection: Description of five cases treated in two Italian institutions and review of the literature. Ann Vasc Surg. 67: 565.e17-24
4. Bruno G, De Stefani A, Barone M, Costa G, Saccomanno S, Gracco A. The validity of panoramic radiograph as a diagnostic method for elongated styloid process: A systematic review. Cranio. 2019. p. 1-8
5. Cohen MA, Evins AI, Lapadula G, Arko L, Stieg PE, Bernardo A. The rectus capitis lateralis and the condylar triangle: Important landmarks in posterior and lateral approaches to the jugular foramen. J Neurosurg. 2017. 127: 1398-406
6. Dashti SR, Nakaji P, Hu YC, Frei DF, Abla AA, Yao T. Styloidogenic jugular venous compression syndrome: Diagnosis and treatment: Case report. Neurosurgery. 2012. 70: E795-9
7. Eagle WW. Elongated styloid processes: Report of two cases. Arch Otolaryngol. 1937. 25: 584-7
8. Farhat HI, Elhammady MS, Ziayee H, Aziz-Sultan MA, Heros RC. Eagle syndrome as a cause of transient ischemic attacks. J Neurosurg. 2009. 110: 90-3
9. Fusco DJ, Asteraki S, Spetzler RF. Eagle’s syndrome: Embryology, anatomy, and clinical management. Acta Neurochir (Wien). 2012. 154: 1119-26
10. Lee ML, Jee TK, Lee JA, Park K. Postoperative complications of microvascular decompression for hemifacial spasm: Lessons from experience of 2040 cases. Neurosurg Rev. 2016. 39: 151-8
11. Lee S, Park SK, Joo BE, Lee JA, Park K. Vascular complications in microvascular decompression: A survey of 4000 operations. World Neurosurg. 2019. 130: e577-82
12. Li M, Gao X, Rajah GB, Liang J, Chen J, Yan F. Styloidectomy and venous stenting for treatment of styloid-induced internal jugular vein stenosis: A case report and literature review. World Neurosurg. 2019. 30: 129-32
13. Li M, Sun Y, Chan CC, Fan C, Ji X, Meng R. Internal jugular vein stenosis associated with elongated styloid process: Five case reports and literature review. BMC Neurol. 2019. 19: 112
14. Li N, Zhao W, Pu C, Shen J. Delayed hemorrhage following microvascular decompression. Three case reports. Neurol Med Chir (Tokyo). 2007. 47: 186-8
15. Rechtweg JS, Wax MK. Eagle’s syndrome: A review. Am J Otolaryngol. 1998. 19: 316-21
16. Reis CV, Deshmukh V, Zabramski JM, Crusius M, Desmukh P, Spetzler RF. Anatomy of the mastoid emissary vein and venous system of the posterior neck region: Neurosurgical implications. Neurosurgery. 2007. 61: 193-201
17. Sindou M, Mercier P. Microvascular decompression for hemifacial spasm: Outcome on spasm and complications. A review. Neurochirurgie. 2018. 64: 106-16
18. Xia L, Liu MX, Zhong J, Dou NN, Li B, Sun H. Fatal complications following microvascular decompression: Could it be avoided and salvaged?. Neurosurg Rev. 2017. 40: 389-96
19. Zamboni P, Scerrati A, Manegatti E, Galeotti R, Lapparelli M, Traina L. The eagle jugular syndrome. BMC Neurol. 2019. 19: 333
20. Zhao H, Zhang X, Tang Y, Zhang Y, Ying TT, Zhu J. Operative complications of microvascular decompression for hemifacial spasm: Experience of 1548 cases. World Neurosurg. 2017. 107: 559-64