- Trauma Research Center, Shahid Rajaee (Emtiaz) Trauma Hospital, Department of Neurosurgery, Shiraz University of Medical Sciences, Shiraz, Iran.
- Medical School, Shiraz University of Medical Sciences, Shiraz, Iran.
- Department of Epidemiology, School of Health, Shiraz University of Medical Sciences, Shiraz, Iran.
Correspondence Address:
Mohammad Aryaie, Department of Epidemiology, School of Health, Shiraz University of Medical Sciences, Shiraz, Iran.
DOI:10.25259/SNI_932_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Hosseinali Khalili1, Saeed Abdollahifard2, Amin Niakan1, Mohammad Aryaie3. The effect of Vitamins C and E on clinical outcomes of patients with severe traumatic brain injury: A propensity score matching study. 25-Nov-2022;13:548
How to cite this URL: Hosseinali Khalili1, Saeed Abdollahifard2, Amin Niakan1, Mohammad Aryaie3. The effect of Vitamins C and E on clinical outcomes of patients with severe traumatic brain injury: A propensity score matching study. 25-Nov-2022;13:548. Available from: https://surgicalneurologyint.com/surgicalint-articles/12026/
Abstract
Background: The aim of this study was to assess the effect of Vitamins C and E on mortality, intensive care unit (ICU) length of stay, and Glasgow Outcome Scale-Extended (GOS-E) score of traumatic brain injury (TBI) patients.
Methods: Using data from records of patients in a retrospective cohort study, we included 1321 TBI patients, 269 treated and 1052 untreated, aged over 18 years with information on exposure (i.e., Vitamins C and E) and confounders. Age, Glasgow Coma Scale, pupil status, Rotterdam classification, blood sugar, blood pressure, international normalized ratio, and comorbidity of patients were considered as the confounding factors. Endpoints were GOS-E on follow-up, mortality, and ICU length of stay. Propensity score matching was performed to adjust the confounders.
Results: Based on the average treatment effect estimates, the use of Vitamins C and E reduced the risk of mortality (risk difference [RD]: −0.07; 95% confidence interval [CI]: −0.14–−0.003) and reduced the length of ICU stay (RD -1.77 95% CI:-3.71-0.16). Furthermore, our results showed that GOS-E was improved significantly (RD: 0.09, 95% CI : 0.03-0.16).
Conclusion: Our study suggests that using Vitamins C and E could decrease mortality and length of ICU stay and improve the GOS-E score and functions of the patients with severe TBI. As they are safe and inexpensive medications, they can be used in routine practice in ICUs to improve the outcomes of TBI patients.
Keywords: Ascorbic acid, Traumatic brain injury, TBI, Vitamin C, Vitamin E
INTRODUCTION
Traumatic brain injury (TBI) is an acute injury to the skull caused by external sources that can be classified into mild, moderate, and severe according to the patient’s condition.[
MATERIALS AND METHODS
This study was a retrospective cohort and was prepared according to the Strengthening the Reporting of Observational Studies in Epidemiology guideline.[
We considered the follow-up time for the GOS-E score to be 6 months after discharge from the hospital. Given the availability of these drugs in our hospital and the expert opinion of attending physicians regarding the patients’ conditions, Vitamins C and E were prescribed. The prescribed dosage of Vitamins C and E in the ICU setting was 3 days of intravenous administration of both vitamin C (500 mg) and vitamin E (100 units) started within 5 days after TBI.
Statistical analysis
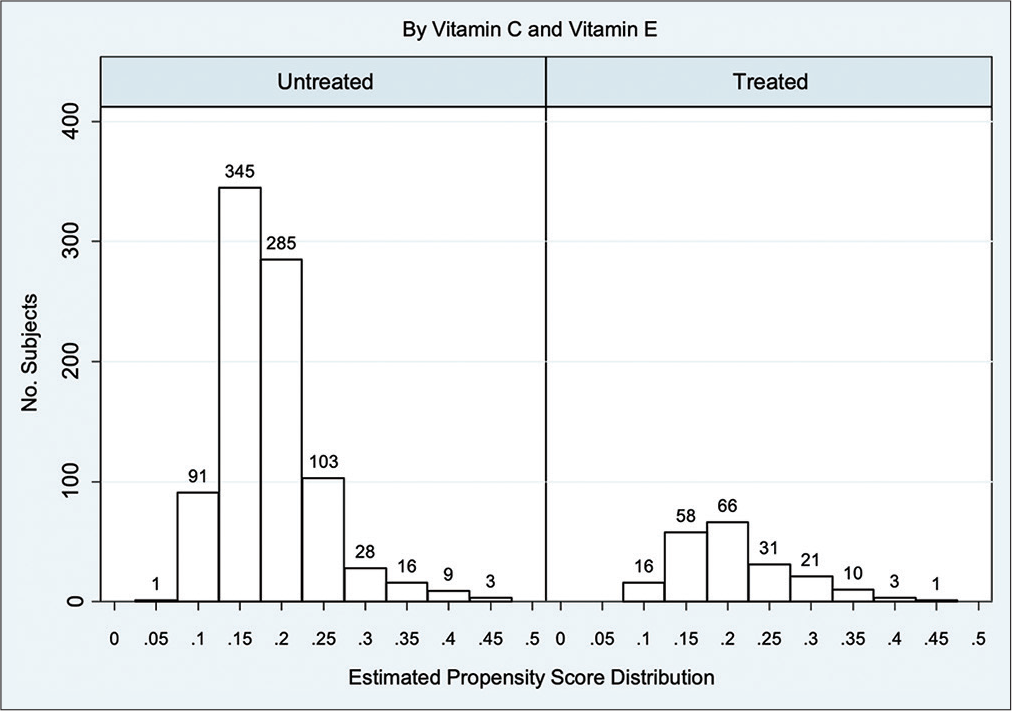
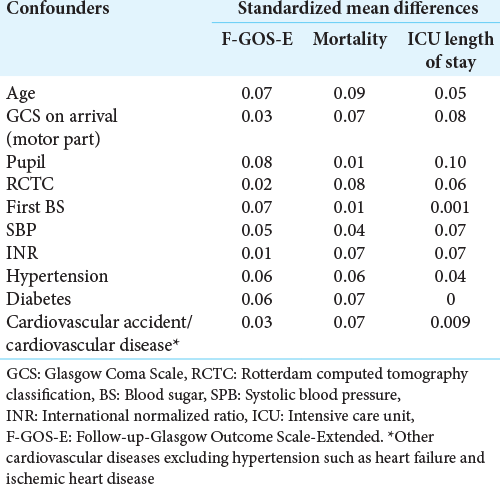
We performed PSM using a logistic regression model to balance the potential confounders, including the admission age, GCS on arrival (motor part), pupil condition, Rotterdam CT classification, blood sugar, systolic blood pressure, international normalized ratio, and preexisting comorbidities condition (i.e., diabetes, hypertension, and other cardiovascular diseases), between treated and untreated patients. We assessed PS matching (one to many matching) between treated and untreated patients using a caliper of 0.05, meaning that the PS could have varied by 5% for the two members of a matched set.[
After PS matching, we estimated the average treatment effect (ATE) using the risk difference (RD) for the outcome of interest, and the 95% confidence intervals (CIs) were derived using robust standard error.
Ethics
The ethics committee of Shiraz University of Medical Sciences approved this study to be conducted with registry number IR sums.med.rec.1400.600.
RESULTS
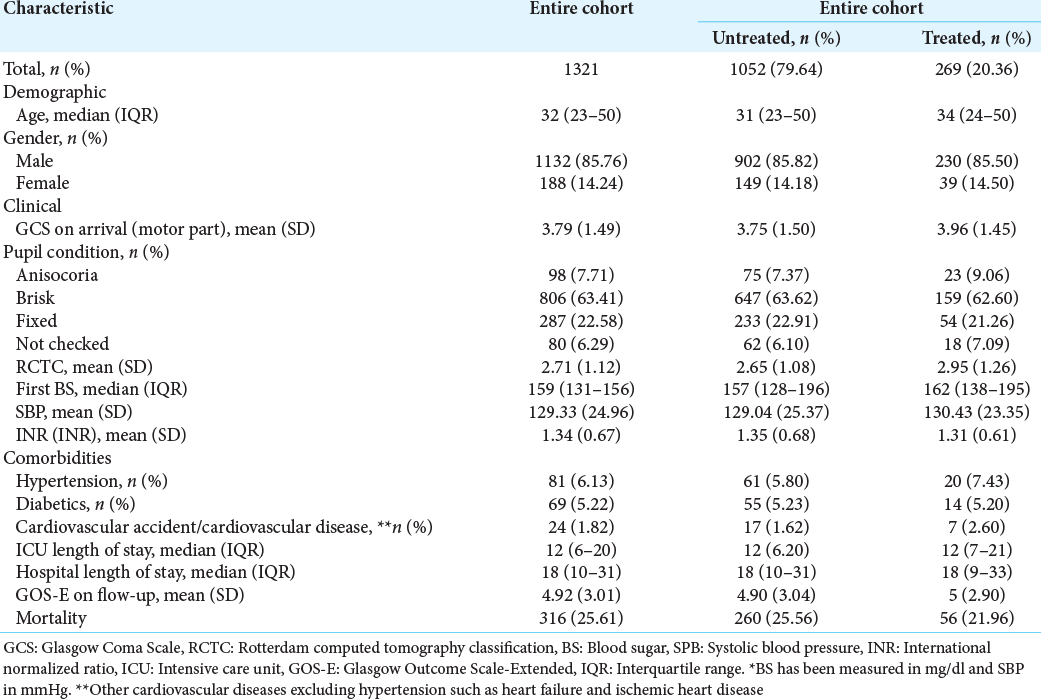
Among the total of 1321 patients included in the study, we identified 269 (20.36%) patients treated with Vitamins C and E. The baseline characteristics of patients treated and untreated with Vitamins C and E are presented in
The median (interquartile range [IQR]) age of the patients untreated and treated with C and E at the entry into the study was 31 (IQR: 23–50) and 34 years (IQR: 24–50). Patients who were untreated with Vitamins C and E were more likely to be dead, with a lower rate of cardiovascular diseases (except hypertension such as heart failure and ischemic heart disease) and hypertension, but they had a higher international normalized ratio score [
Based on ATE estimates, the use of Vitamins C and E reduced the risk of mortality (RD: −0.07; 95% CI: −0.14–−0.003) and reduced the ICU length of stay (RD: −1.77; 95% CI: −3.71–0.16). Furthermore, our results showed that GOS-E was improved significantly (RD: 0.09, 95% CI : 0.03-0.16).
DISCUSSION
To the best of our knowledge, this is the first study with a large number of patients and PSM that investigates the effect of Vitamins C and E on clinical outcomes of TBI patients.
Our results showed that Vitamins C and E could remarkably reduce the risk of mortality and ICU length of stay and help improve the GOS-E score of patients as a clinical outcome index.
Corticosteroid Randomization after Significant Head Injury and the International Mission for Prognosis and Analysis of Clinical Trials in TBI are two models that were developed in 2008 for the prediction of TBI patients’ prognosis.[
By taking these factors into account, as we wanted to divide the patients into cases and controls, we considered the abovementioned baseline characteristic as a cofounder of our study and performed a PSM to eliminate systematic differences. Originally introduced by Rosenbaum and Rubin in 1983, PSM is a technique for ensuring that in observational studies, the distributions of baseline covariates are approximately the same for both treatment and control groups. Based on the similar characteristics of these two groups, a setting that mimics randomized clinical trials is simulated.[
Preclinical studies on animal models demonstrated that Vitamin C could decrease the mortality rate in rats with hypoxic brain damage and vasospasm following subarachnoid hemorrhage. Three out of four studies that evaluated the effect of Vitamin C on TBI in rats[
The only clinical study was carried out by Razmkon et al. During this double-blinded controlled trial, they administrated low (500 mg/day for 7 days intravenously) and high (10 g/day for the 1st and 4th days and 4 g/day for other 3 days) doses of Vitamin C. Patients with a head injury, GCS under 8, and radiology imaging in favor of diffuse axonal injury were included and significant renal or liver failure, glucose-6-phosphate dehydrogenase deficiency (G6PDD), and previous central nervous system lesions were the exclusion criteria. Duration of hospital stay (29.7% vs. 26.9% and 30.4% in low dose and high dose of Vitamin C, respectively) and mortality rate (29.7% vs. 34.6% and 30.4% in low dose and high dose of Vitamin C, respectively) were not significantly different between the control group and the patients who were administrated Vitamin C. Interestingly, perilesional edema was stable or reduced in 68% of patients receiving a high dose of Vitamin C.[
There was some difference, regardless of design, between our study and Razmkon’s study that is worth mentioning. To begin with, our study had a sample size 13-fold greater than this study. Second, although this study included a control group of patients, the controls were not matched to the treatment arm in terms of confounding factors. Third, the severe patients have been defined by Razmkon et al. as patients with a GCS score of less than 8 while our study considered patients with AIS-head more than and equal to 3 without AIS more than 3 in other regions. Last but not least, there was a difference in vitamin dosage. The dose of Vitamins C and E in our study was similar to the low dose of Vitamin C and Vitamin E in the Razmkon et al. study, but they prescribed these two drugs separately for 7 days, and we administered them simultaneously for 3 days.
The role of Vitamin E has been described as a booster of cognitive disturbance improvement following TBI.[
Mechanism and rationale for administration of Vitamins C and E
In traumatic brain injuries and the following secondary insults, oxidative stress and release of free radicals are significantly increased.[
Costs and adverse effects
To speak economically, it has been reported that supplementary administration of AA during pregnancy will cost about 20$, and monthly administration of Vitamin E costs 5$.[
Limitation
Our study was limited by the unavailability of these two vitamins in our center. The retrospective nature of our study and being conducted in a single center are the other limitations. Since we estimated the effect of Vitamins C and E from an observational study, instead of a well-defined randomized clinical trial, some unknown confounders might have also distorted our results.
CONCLUSION
Our study suggests that administering Vitamins C and E may decrease the mortality and length of ICU stay and improve the GOS-E score and functions of the patients with severe TBI. This study suggests that these two vitamins may have neuroprotective effects. Besides the fact that they are safe and inexpensive, they can be used in routine practice to decrease the burden of TBI on the patients. For consolidation of our results, further randomized clinical trials with different doses and both separate and combined administration of Vitamins C and E are needed.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Publication of this article was made possible by the James I. and Carolyn R. Ausman Educational Foundation.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Abdullah M, Attia FN, editors. Vitamin C (Ascorbic Acid). StatPearls. Treasure Island: StatPearls Publishing; 2022. p. Available from: https://www.ncbi.nlm.nih.gov/books/NBK499877 [Last accessed on 2021 Jun 15]
2. Aiguo W, Zhe Y, Gomez-Pinilla F. Vitamin E protects against oxidative damage and learning disability after mild traumatic brain injury in rats. Neurorehabil Neural Repair. 2010. 24: 290-8
3. Arango MF, Bainbridge D, editors. Magnesium for acute traumatic brain injury. Cochrane Database Syst Rev. 2008. p. Cd005400
4. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011. 46: 399-424
5. Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011. 10: 150-61
6. Balestreri M, Czosnyka M, Chatfield DA, Steiner LA, Schmidt EA, Smielewski P. Predictive value of Glasgow Coma Scale after brain trauma: Change in trend over the past ten years. J Neurol Neurosurg Psychiatry. 2004. 75: 161-2
7. Brau RH, García-Castiñeiras S, Rifkinson N. Cerebrospinal fluid ascorbic acid levels in neurological disorders. Neurosurgery. 1984. 14: 142-6
8. Clarke MW, Burnett JR, Croft KD. Vitamin E in human health and disease. Crit Rev Clin Lab Sci. 2008. 45: 417-50
9. Clifton GL, Lyeth BG, Jenkins LW, Taft WC, DeLorenzo RJ, Hayes RL. Effect of D, alpha-tocopheryl succinate and polyethylene glycol on performance tests after fluid percussion brain injury. J Neurotrauma. 1989. 6: 71-81
10. Comitato R, Ambra R, Virgili F. Tocotrienols: A family of molecules with specific biological activities. Antioxidants (Basel). 2017. 6: 93
11. Conte V, Uryu K, Fujimoto S, Yao Y, Rokach J, Longhi L. Vitamin E reduces amyloidosis and improves cognitive function in Tg2576 mice following repetitive concussive brain injury. J Neurochem. 2004. 90: 758-64
12. Di Pietro V, Yakoub KM, Caruso G, Lazzarino G, Signoretti S, Barbey AK. Antioxidant therapies in traumatic brain injury. Antioxidants (Basel). 2020. 9: 260
13. Dobrovolny J, Smrcka M, Bienertova-Vasku J. Therapeutic potential of Vitamin E and its derivatives in traumatic brain injury-associated dementia. Neurol Sci. 2018. 39: 989-98
14. Etminan M, Nazemipour M, Mansournia MA, Candidate MS. Potential biases in studies of acid-suppressing drugs and COVID-19 infection. Gastroenterology. 2021. 160: 1443-6
15. Gardner AJ, Zafonte R, Aminoff MJ, Boller F, Swaab DF, editors. Neuroepidemiology of traumatic brain injury. Handbook of Clinical Neurology. Ch. 12. Netherlands: Elsevier; 2016. p. 207-23
16. Gennarelli TA, Wodzin E. AIS 2005: A contemporary injury scale. Injury. 2006. 37: 1083-91
17. Gooch CL, Pracht E, Borenstein AR. The burden of neurological disease in the United States: A summary report and call to action. Ann Neurol. 2017. 81: 479-84
18. Harrison FE, May JM. Vitamin C function in the brain: Vital role of the ascorbate transporter SVCT2. Free Radic Biol Med. 2009. 46: 719-30
19. Head B, Du JL, Tanguay R, Kioussi C, Traber M. Vitamin E is necessary to protect neural crest cells in developing zebrafish embryos. Curr Dev Nutr. 2020. 4: 1209
20. Hernán MA, Robins JM, editors. Causal Inference: What if, Boca Raton: Chapman and Hall/CRC. 2020. p.
21. Hill A, Wendt S, Benstoem C, Neubauer C, Meybohm P, Langlois P. Vitamin C to improve organ dysfunction in cardiac surgery patients-review and pragmatic approach. Nutrients. 2018. 10: 974
22. Inci S, Ozcan OE, Kilinç K. Time-level relationship for lipid peroxidation and the protective effect of alpha-tocopherol in experimental mild and severe brain injury. Neurosurgery. 1998. 43: 330-5 discussion 335-6
23. Ishaq GM, Saidu Y, Bilbis LS, Muhammad SA, Jinjir N, Shehu BB. Effects of α-tocopherol and ascorbic acid in the severity and management of traumatic brain injury in albino rats. J Neurosci Rural Pract. 2013. 4: 292-7
24. Jiang Q. Natural forms of Vitamin E: Metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic Biol Med. 2014. 72: 76-90
25. Kangisser L, Tan E, Bellomo R, Deane AM, Plummer MP. Neuroprotective properties of Vitamin C: A scoping review of pre-clinical and clinical studies. J Neurotrauma. 2021. 38: 2194-205
26. Khodamoradi F, Nazemipour M, Mansournia N, Yazdani K, Khalili D, Mansournia MA. The effects of smoking on metabolic syndrome and its components using causal methods in the Iranian population. Int J Prev Med. 2021. 12: 118
27. Kowalski RG, Hammond FM, Weintraub AH, Nakase-Richardson R, Zafonte RD, Whyte J. Recovery of consciousness and functional outcome in moderate and severe traumatic brain injury. JAMA Neurol. 2021. 78: 548-57
28. Leichtle SW, Sarma AK, Strein M, Yajnik V, Rivet D, Sima A. High-dose intravenous ascorbic acid: Ready for prime time in traumatic brain injury?. Neurocrit Care. 2020. 32: 333-9
29. Lin JL, Huang YH, Shen YC, Huang HC, Liu PH. Ascorbic acid prevents blood-brain barrier disruption and sensory deficit caused by sustained compression of primary somatosensory cortex. J Cereb Blood Flow Metab. 2010. 30: 1121-36
30. Maekawa T, Uchida T, Nakata-Horiuchi Y, Kobayashi H, Kawauchi S, Kinoshita M. Oral ascorbic acid 2-glucoside prevents coordination disorder induced via laser-induced shock waves in rat brain. PLoS One. 2020. 15: e0230774
31. Medina JG. Vitamin E. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557737 [Last accessed on 2022 Jan ???].
32. Perel P, Arango M, Clayton T, Edwards P, Komolafe E. Predicting outcome after traumatic brain injury: Practical prognostic models based on large cohort of international patients. BMJ. 2008. 336: 425-9
33. Pérez-Rodríguez L, Redondo T, Ruiz-Mata R, Camacho C, Moreno-Rueda G, Potti J. Vitamin E supplementation-but not induced oxidative stress-influences telomere dynamics during early development in wild passerines. Front Ecol Evol. 2019. 7:
34. Peterson ABXu LDaugherty JBreiding MJ. Surveillance Report of Traumatic Brain Injury-Related Emergency Department Visits, Hospitalizations, and Deaths, United States, 2014. Available from: https://www.cdc.gov/traumaticbraininjury/pdf/TBI-Surveillance-Report-FINAL_508.pdf [Last accessed on 2022 Oct 09].
35. Polidori MC, Mecocci P, Frei B. Plasma Vitamin C levels are decreased and correlated with brain damage in patients with intracranial hemorrhage or head trauma. Stroke. 2001. 32: 898-902
36. Rajajee V, Moreira ME, Rabinstein AA, editors. Management of Acute Moderate and Severe Traumatic Brain Injury. Waltham: Up to Date. 2019. p.
37. Razmkon A, Sadidi A, Sherafat-Kazemzadeh E, Mehrafshan A, Jamali M, Malekpour B. Administration of Vitamin C and Vitamin E in severe head injury: A randomized double-blind controlled trial. Clin Neurosurg. 2011. 58: 133-7
38. Rice ME, Lee EJ, Choy Y. High levels of ascorbic acid, not glutathione, in the CNS of anoxia-tolerant reptiles contrasted with levels in anoxia-intolerant species. J Neurochem. 1995. 64: 1790-9
39. Richard B. ALSUntangled 55: Vitamin E (α-tocopherol). Amyotroph Lateral Scler Frontotemporal Degener. 2021. 22: 154-60
40. Roberts I, Yates D, Sandercock P, Farrell B, Wasserberg J, Lomas G. Effect of intravenous corticosteroids on death within 14 days in 10008 adults with clinically significant head injury (MRC CRASH trial): Randomised placebo-controlled trial. Lancet. 2004. 364: 1321-8
41. Robertson CS, Hannay HJ, Yamal JM, Gopinath S, Goodman JC, Tilley BC. Effect of erythropoietin and transfusion threshold on neurological recovery after traumatic brain injury: A randomized clinical trial. JAMA. 2014. 312: 36-47
42. Servadei F, Murray GD, Penny K, Teasdale GM, Dearden M, Iannotti F. The value of the “worst” computed tomographic scan in clinical studies of moderate and severe head injury. European brain injury consortium. Neurosurgery. 2000. 46: 70-5 discussion 75-77
43. Sivandzade F, Alqahtani F, Cucullo L. Traumatic brain injury and blood-brain barrier (BBB): Underlying pathophysiological mechanisms and the influence of cigarette smoking as a premorbid condition. Int J Mol Sci. 2020. 21: 2721
44. Steyerberg EW, Mushkudiani N, Perel P, Butcher I, Lu J, McHugh GS. Predicting outcome after traumatic brain injury: Development and international validation of prognostic scores based on admission characteristics. PLoS Med. 2008. 5: e165 discussion e165
45. Surai PF, editors. Natural Antioxidants in Avian Nutrition and Reproduction: Nottingham: Nottingham University Press. 2002. p.
46. Taylor CA, Bell JM, Breiding MJ, Xu L. Traumatic brain injury-related emergency department visits, hospitalizations, and deaths-United States, 2007 and 2013. MMWR Surveill Summ. 2017. 66: 1-16
47. Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ. Strengthening the reporting of observational studies in epidemiology (STROBE): Explanation and elaboration. PLoS Med. 2007. 4: e297
48. Wang KW, Wang HK, Chen HJ, Liliang PC, Liang CL, Tsai YD. Simvastatin combined with antioxidant attenuates the cerebral vascular endothelial inflammatory response in a rat traumatic brain injury. BioMed Res Int. 2014. 2014: 910260
49. Yang J, Han Y, Ye W, Liu F, Zhuang K, Wu G. Alpha tocopherol treatment reduces the expression of Nogo-A and NgR in rat brain after traumatic brain injury. J Surg Res. 2013. 182: e69-77
50. Yieh L, McEvoy CT, Hoffman SW, Caughey AB, MacDonald KD, Dukhovny D. Cost effectiveness of vitamin c supplementation for pregnant smokers to improve offspring lung function at birth and reduce childhood wheeze/asthma. J Perinatol. 2018. 38: 820-7