- Department of Anatomical Sciences, Tabriz University of Medical Sciences, Tabriz, Iran
- Department of Anatomical Sciences, Faculty of Medicine, Lorestan University of Medical Sciences, Khorramabad, Iran
Correspondence Address:
Amir A. Khaki
Department of Anatomical Sciences, Tabriz University of Medical Sciences, Tabriz, Iran
DOI:10.4103/2152-7806.185006
Copyright: © Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Teimori F, Khaki AA, Rajabzadeh A, Roshangar L. The effects of 30 mT electromagnetic fields on hippocampus cells of rats. Surg Neurol Int 29-Jun-2016;7:70
How to cite this URL: Teimori F, Khaki AA, Rajabzadeh A, Roshangar L. The effects of 30 mT electromagnetic fields on hippocampus cells of rats. Surg Neurol Int 29-Jun-2016;7:70. Available from: http://surgicalneurologyint.com/surgicalint_articles/effects-30-mt-electromagnetic-fields-hippocampus-cells-rats/
Abstract
Background:Despite the use of electromagnetic waves in the treatment of some acute and chronic diseases, application of these waves in everyday life has created several problems for humans, especially the nerve system. In this study, the effects of 30mT electromagnetic fields (EMFs) on the hippocampus is investigated.
Methods:Twenty-four 5-month Wistar rats weighing 150–200 g were divided into two groups. The experimental group rats were under the influence of an EMF at an intensity of 3 mT for approximately 4 hours a day (from 8 AM to 12 PM) during 10 weeks. After the hippocampus was removed, thin slides were prepared for transmission electron microscope (TEM) to study the ultrastructural tissue. Cell death detection POD kits were used to determine the apoptosis rate.
Results:The results of the TEM showed that, in the hippocampus of the experimental group, in comparison to the control group, there was a substantial shift; even intracellular organelles such as the mitochondria were morphologically abnormal and uncertain. The number of apoptotic cells in the exposed group compared to the control group showed significant changes.
Conclusions:Similar to numerous studies that have reported the effects of EMFs on nerves system, it was also confirmed in this lecture. Hence, the hippocampus which is important in regulating emotions, behavior, motivation, and memory functions, may be impaired by the negative impacts of EMFs.
Keywords: Electromagnetic fields, hippocampus, rats
INTRODUCTION
Electromagnetic radiation is a combination of an electric field and a magnetic field perpendicular to each other and in the direction perpendicular to the two fields which is distributed in space. Unlike other waves, electromagnetic waves pass through vacuum. In general, waves including electromagnetic radiation consist of four main characteristics of wavelength, frequency, speed, and amplitude. High-frequency photons have considerable energy to break chemical bonds, and this part of the spectrum is called as ionizing. A photon's energy to separate the chemical bonds is too low at low frequency, which is called the non-ionizing part of the electromagnetic spectrum. Therefore, the biological effects of electromagnetic waves can be divided into ionizing and non-ionizing parts. Non-ionizing radiation can have adverse effects on human health such as headache, stress, fatigue, and anxiety.[
It's reported that exposure by electromagnetic fields in children may be led to the increased white blood cells and consequently the formation of leukemia.[
Moreover, according to some evidence, the hippocampus plays a role in converting recent memory to long-term memory, and any damage to it causes severe memory impairment. Referring to the previous studies, neurological disorders caused by electromagnetic waves involve memory loss, lethargy, insomnia, and depression. Previous investigations have shown mild side effects such as headaches, dizziness, temporary amnesia, sleep disorders, and reduction of learning ability, as well as remarkable effects such as leukemia, and gene mutation, chromosomal abnormalities in humans and animals.[
SUBJECTS AND METHODS
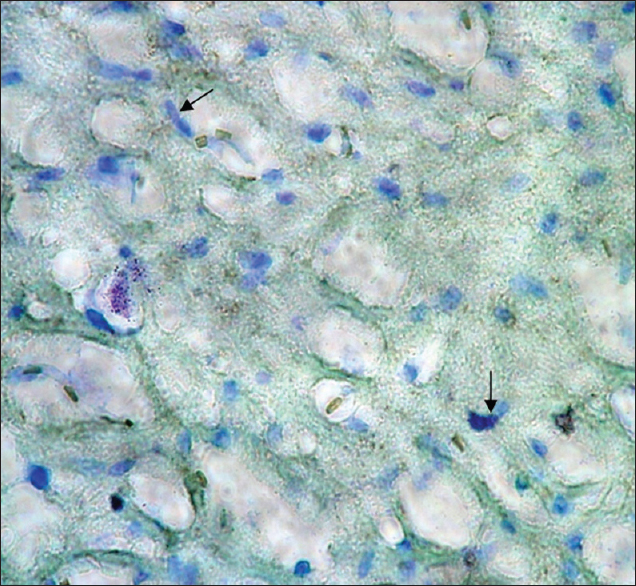
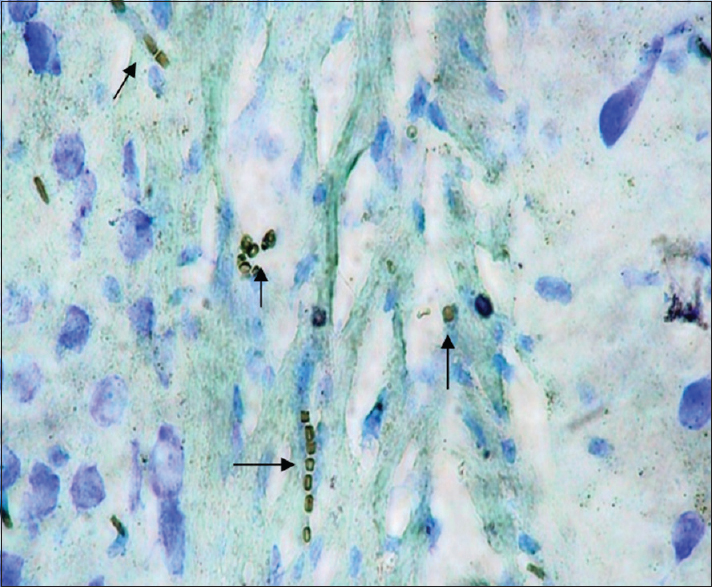
Twenty-four 5-month Wistar rats weighing 150–200 g were used in this experimental study. The rats were collectively kept in a standard condition at a temperature of 2 ± 24°C, 12 h light and 12 h of darkness, and humidity of 50–70 in a room with continuous ventilation inside metal cages. Food and water were given to the rats without any restriction. The rats were assigned to two groups, that is, experimental and control groups, with 12 rats in each group. The experimental group rats were exposed to EMF at an intensity of 3 mT for approximately 4 h a day (from 8 AM to 12 PM) for 10 weeks. An EMF generator device, producing a field of 3 mT, designed and manufactured in Medical Physics, was used. The generator was made of according to the Helmholtz theory. With regard to this selection, issues such as the need for achieving a uniform field and with certain intensity and different constraints like the need for a suitable place for rats living inside the machine were the determinative. Considering to the generator ampere consumption and its long time use during the day, to avoid overheating the device proper ventilation, the ventilation system was installed on to. Generally, the generator consists of 2 windings of opposite direction which produce a uniform field in the center of the device where animals are located. A uniform field was produced between the windings due to passing electricity with alternating current and frequency of 50 Hz. At the end of the experimental period, at first, the rats were anesthetized by a piece of cotton soaked in chloroform in a plastic container in the package. After stopping the heartbeat, we proceeded to remove the head and skull. After the brain became visible, we took the brain out of the skull hole seamlessly and carefully. Then, the hippocampus was removed and washed with 0.1 M phosphate buffer at a pH of 7.4. Next, secondary stabilization was performed by 1% osmium tetroxide. After dehydration using ethanol, the samples were made transparent by using propylene oxide. In the last phase, the samples were embedded in resin. Uranyl acetate and lead nitrate using transmission electron microscopy (TEM) (LEO, 200 Germany) were applied for coloring of toluidine blue semi-thin slices as well as thin slices. Cell death detection POD kits (Roche Company made in Germany) were used for determining the apoptosis rate. Coloring was performed by toluidine blue. Using these kits, apoptotic cell nucleus turned brown, while the normal cell nuclei retained the toluidine blue color. In this section, the hippocampus samples were stained by TUNEL techniques, therefore, apoptotic cells became visible as golden brown.
RESULTS
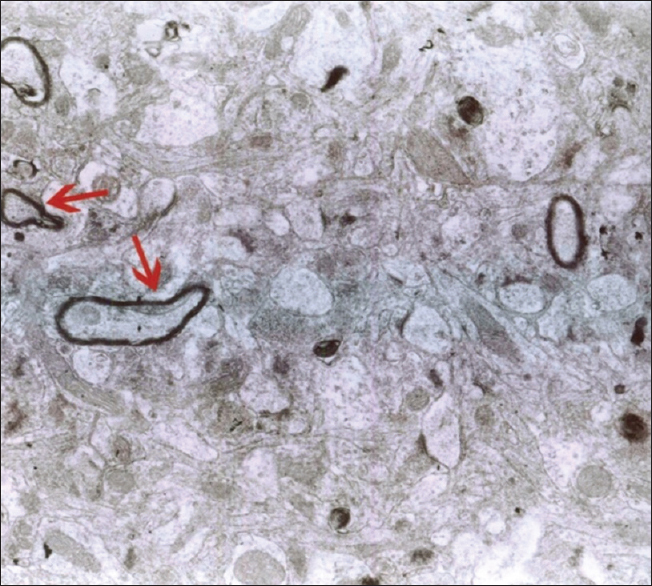
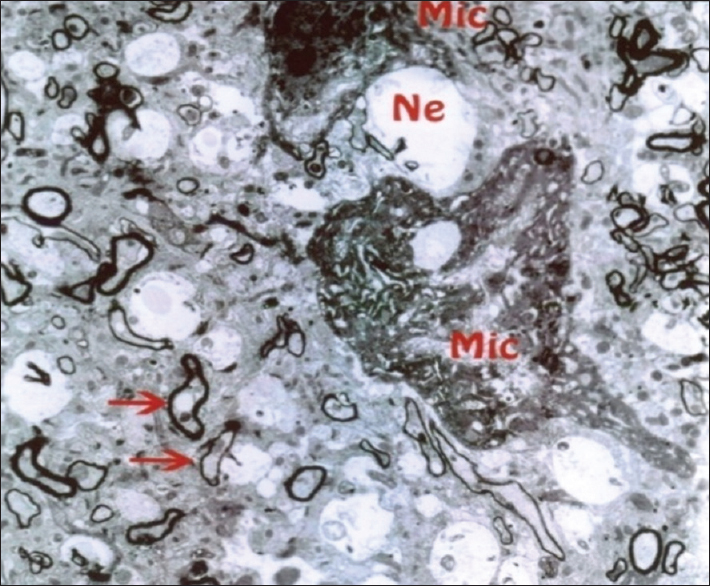
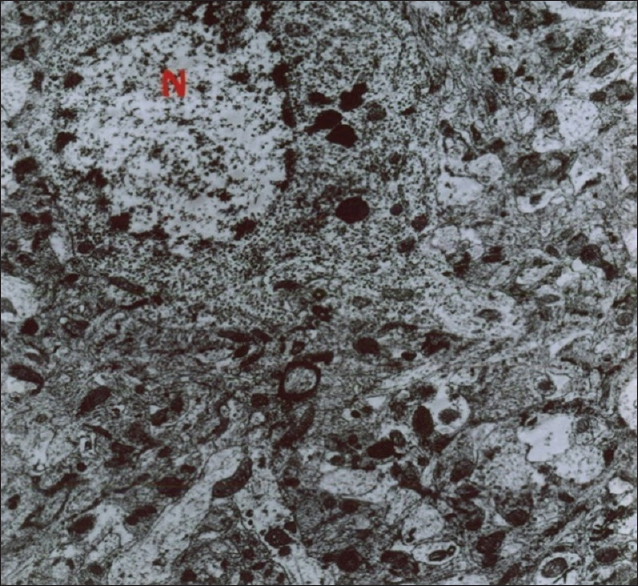
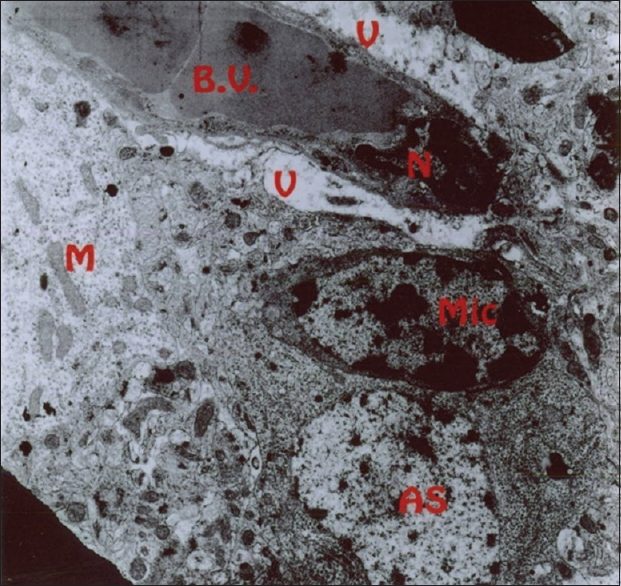
As mentioned previously, the aim of this study was to evaluate the ultrastructural and histochemical changes resulting from electromagnetic waves at a frequency of 50 Hz and an intensity of 3 mT on the hippocampus of rats’ brain after exposure to the EMF. Therefore, findings of the effects of EMFs on hippocampal tissue among the groups exposed to the EMF, considering the mentioned terms and conditions, and the control group without exposure to EMFs have been investigated. The results are presented in two parts, that is, ultrastructural changes and histochemical examination. Ultrastructural changes: As seen in
DISCUSSION
The results of the Bio Institute group research in 2009 showed that electromagnetic radiations from cell phones’ high-pressure lines, telecommunications stations, and many other sources emitting electromagnetic radiation are acceptable, permissible, and compatible to the standards of Federal Communications Commission (FCC) in daily lives of humans. These radiations do not have health risks. However, when humans are exposed for hundreds and thousands of times, and sometimes permanently exposed to electromagnetic radiation, absorption level of radiations exceed the standard limit, and thus their adverse effects develop. The results of this study showed that exposure to electromagnetic fields of 50 Hz with 3 mT intensity leads to a significant increase in apoptotic cells. It should be noted that these cells are mainly visible in the hippocampus glial cells. TUNEL technique in the present study clearly showed apoptotic glial cells. It seems that reduction in the number of glial cells and neurons in confirming the occurrence of apoptosis in neurons and glial cells, which can be due to the field impact on cell programmed death and its incidence, should be considered. These findings are consistent with studies conducted by Salford to examine the relationship between pathological leakage of albumin from the blood–brain barrier and neuronal damage by pulsed microwaves emitted from mobile. The results of this research revealed a significant destruction of neurons in the cortex, hippocampus, and basal ganglia of rats that were exposed to mobile radiation for 2 hours. Perhaps the cell death observed in this study is due to the production of free radicals in brain tissue neurons of the irradiated mice.[
The current theory is compatible with the studies conducted by Lai and et al. They examined the effects of a magnetic field of 0.01 mT with a frequency of 60 Hz for 24 hours to assess the level of DNA breaks in rats’ brain cells. Results have shown that high-intensity electromagnetic waves lead to increase in apoptosis and necrosis in the brain cells of rats. This increase in mortality may be due to the free radicals produced by electromagnetic waves that results in breaking DNA and nerve cells death.[
Other findings of this study on cerebellum reveal significant decrease in the number of Purkinje cells under the influence of EMF that can be due to their effect on programmed cell death and apoptosis. In addition, some findings show the effect of EMF on calcium homeostasis changes of membrane and in stimulating the synthesis of ornithine decarboxylase as well as an increase in activity that alters the expression of DNA synthesis.[
A similar examination conducted on the kidney structure of rats for 52 days and 1 hour in a day. The rats were exposed to a field of low frequency (9450 MHZ). Due to light microscopic examination and vacuolization, epithelium kidney tubules were observed in the apical and degenerative changes in the glomerulus were realized. Inflation of organelles with membranes of mitochondria and endoplasmic reticulum were observed through the examination by an electron microscope.[
Examination of the proliferating cells shows that the most vulnerable time is during the phase G/S of the cell cycle when transferrin receptors are expressed and too much iron is penetrated. Free radicals produce large amounts of hydrogen peroxide in the presence of iron via the Fenton reaction. Cells with high metabolic activity produce large amounts of hydrogen peroxide through the mitochondrial electron transport, and therefore become more vulnerable to the effects of EMF. On the other hand, the negative effects of EMF depend on the cells iron storage ability in the form of ferritin. For example, liver cells can be less susceptible to the effects of EMF even when they are having a lot of iron input because they contain lots of ferritin. Cancer cells are realized by transferrin receptors on the surface, thus they have high iron absorption. The effects of free radicals are very extensive, however, there are four reactions that are associated specifically with cell harassment: Membrane lipids peroxidation, mitochondrial damage, creating crosslinking in proteins, and DNA damage. However, perhaps damaging effects of electromagnetic waves are because of the body's rising temperature and producing free radicals that both agents could be considered as damaging elements to the tissues of the body, especially the nervous system. Therefore, in order to eliminate the negative impacts of these waves on the nervous system, it is recommended that unnecessary and prolonged use of devices generating such waves be avoided.
CONCLUSION
This study concluded that EMFs with any frequency and intensity can be damaging to the nervous system. In this study we found that EMFs by increasing structural changes in cells and apoptosis in them can alter the cells function at every position of it. Therefore, by avoiding the usage of these stimulators, their negative effects can be reduced.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We thank the library and website of medical faculty of Tabriz University to ensure more study.
References
1. Ainsh MC.editors. Physics in medicine and Biology. USA: Mc-Graw-Hill, USA; 1986. p. 296-9
2. Aldrich TE, Easterly CE. Electromagnetic fields and public health. Environ Health Perspect. 1987. 75: 159-71
3. Henry Lai N, Singh P. Magnetic-field–induced DNA strand breaks in brain cells of the rat. Environ Health Perspect. 2004. 112: 687-94
4. Herman D, Suti MD, Michael SH. Hyperthermia potentials as an anti-tumor agent. J Med Sci. 1974. 34: 122-9
5. Ian M, Stephen P. Oxidative stress and cardiovascular diseases: Novel tools give (free) radical insight. J Mol Cell Cardiol. 2009. 47: 372-81
6. . IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Nonionizing radiation, Part 1: Static and extremely low frequency (ELF) electric and magnetic fields. IARC Monogr Eval Carcinog Risks Hum. 2002. p. 1380-1395
7. Joseph F. The role of Free Radicals in the nervous system, Oxidative Stress and Free Damage in Neurology. 2011. p. 1-17
8. Marino AA, Becker RO. Biological effects of extremely low frequency electric and magnetic fields: A review. Physiol Chem Phys. 1977. 9: 131-47
9. Mausset A, Motpeyroux F, Privat A. Effects of radio frequency exposure on the GABAergic system in the rat cerebellum: Clues from semi-quantitative immunohistochemistry. Brain Res. 2001. 912: 33-46
10. McNamee JP, Bellier PV, McLean JR, Marro L, Gajda GB, Thansandote A. DNA damage and apoptosis in the immature mouse cerebellum after acute exposure to a 1 mT, 60 Hz magnetic field. Mutat Res. 2002. 513: 121-33
11. Meinert R, Michaelis J. Meta analyses of studies on the association between electromagnetic fields and childhood cancer. Radiat Environ Biophys. 1996. 35: 118-24
12. Miyakoshi J. Biological responses to extremely low frequency electromagnetic fields. J Dermatol Sci. 2006. 2: 23-30
13. Nergiz Y, Ketani A, Akdag Z, Resitersay A, Celik S. Effect of low-intensity microwave radiation on rat kidney: An ultrastructural study. Turk J Med Sci. 2000. 30: 223-7
14. Pankhurst QA, Connolly J, Jones SK, Dobson J. Applications of magnetic nanoparticles in biomedical. J Phys D. 2003. 36: 167-81
15. Repacholi MH. Low-level exposure to radiofrequency electromagnetic fields: Health effects and research needs. Bioelectromagnetics. 1998. 19: 1-19
16. Repacholi MH, Greenebaum B. Interaction of static and extremely low frequency Electric and magnetic Fields with living system: Health effects and research needs. Bioelectromagnetics. 1999. 20: 133-60
17. Robison JG, Pendleton AR, Murry BK, O’Neill KL. Decrease DNA repair rates and protection from heat induced apoptosis mediated by electromagnetic field exposure. Bioelectromagnetics. 2002. 23: 106-12
18. Sahl J, Mezei G, Kavet R, McMillan A, Silvers A, Sastre A. Occupational magnetic field exposure and cardiovascular mortality in a cohort of electric utility workers. Am J Epidemiol. 2002. 156: 913-8
19. Salford LG, Brun AE, Eberhardt JL, Malmgren L, Persson BR. Nerve cell damage in mammalian brain after exposure to microwaves from GSM mobile phones. Environ Health Perspect. 2003. 111: 881-3
20. Savitz DA, Liao D, Sastre A, Kleckner RC, Kavet R. Magnetic field exposure and cardiovascular disease mortality among electric utility workers. Am J Epidemiol. 1999. 149: 135-42
21. Senthil Kumaran V, Arulmathi K, Srividhya R, Kalaiselvi P. Repletion of antioxidant status by EGCG and retardation of oxidative damage induced macromolecular anomalies in aged rats. Exp Gerontol. 2008. 43: 176-83
22. Simonian NA, Coyle JT. Oxidative stress in neurodegenerative diseases. Annu Rev Pharmacol Toxicol. 1996. 3: 83-106
23. Soleimani R, Allahvaisie O, Khaki AA. Electromagnetic field effects on rat's cerebella ultrastructure. Sci J Kurdistan Univ Med Sci. 2006. 11: 1-9
24. Stevens RG, Davies S. The melatonin hypothesis: Electric power and breast cancer. Environ Health Prespect. 1996. 104: 135-40
25. Takahashi S, Inaguma S, Cho YM, Imaida K, Wang J, Fujiwara O, Shirai T. Lack of mutation induction with exposure to 1.5 GHz electromagnetic near fields used for cellular phones in brains of Big Blue mice. Cancer Res. 2002. 62: 1956-60
26. Tenforde TS. Biological interaction of extremely-low-frequency electric and magnetic fields. Biochem Bioenergetics. 1991. 25: 1-17
27. Vanessa J, Philippe L, Marylene C, Sylvie B, Catherine Y. No apoptosis is induced in rat cortical neurons exposed to GSM phone fields. Bioelectromagnetic. 2006. 28: 115-21
28. Wei M, Guizzetti M, Yost M, Costa LG. Exposure to 60-Hz magnetic fields and proliferation of human astrocytoma cells in vitro. Toxicol Appl Pharmacol. 2000. 162: 166-76
29. Xie Y, Jiang HH, Zhang GB, Yu JH. Effect of microwave irradiation on neurocyte mitochondrial ultrastructure and mtTFA mRNA expression in rat's cerebral cortex and hippocampus. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2004. 22: 104-7