- Department of Otorhinolaryngology, Afsin State Hospital, Kahramanmaras, Turkey
- Department of Otorhinolaryngology, Faculty of Medicine, Gazi University, Besevler, Ankara, Turkey
- Department of Pathology, Faculty of Medicine, Gazi University, Besevler, Ankara, Turkey
Correspondence Address:
Ayca Ant
Department of Otorhinolaryngology, Faculty of Medicine, Gazi University, Besevler, Ankara, Turkey
DOI:10.4103/2152-7806.165765
Copyright: © 2015 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ant A, Karamert R, Kulduk G, Özgür Ekinci, Tutar H, Nebil Göksu. The effects of sodium-2-mercaptoethanesulfonate application on the neural and neurovascular tissues: An experimental animal study. Surg Neurol Int 18-Sep-2015;6:150
How to cite this URL: Ant A, Karamert R, Kulduk G, Özgür Ekinci, Tutar H, Nebil Göksu. The effects of sodium-2-mercaptoethanesulfonate application on the neural and neurovascular tissues: An experimental animal study. Surg Neurol Int 18-Sep-2015;6:150. Available from: http://surgicalneurologyint.com/surgicalint_articles/the-effects-of-sodium%e2%80%912%e2%80%91mercaptoethanesulfonate-application-on/
Abstract
Background:Sodium-2-mercaptoethanesulfonate (MESNA) is a protective agent that is also used as “a chemical dissector” in various surgical fields. The aim of this study is to evaluate the toxic effects of MESNA on neural and neurovascular structures based on a morphological analysis and examine its safety in neurotological applications.
Methods:Three groups of guinea pigs were used as subjects. MESNA solution (50 and 100%) and saline solution were applied to the subarachnoid space over the brain tissue via a middle fossa approach of study and control groups, respectively. Effects of MESNA were assessed by means of light microscope. McNemar Chi-square test was used to evaluate the histopathological findings. Statistical significance of P
Results:No morphological changes were observed on vascular and neural structures in the study groups in both concentrations, compared to the control group.
Conclusions:On a morphological basis, a single application of MESNA does not cause any morphological changes that indicate a toxicity in neural and neurovascular structures.
Keywords: Neurotologic surgery, neurotoxicity, sodium-2-mercaptoethanolsulfonate
INTRODUCTION
Sodium-2-mercaptoethanesulfonate (MESNA) is the sodium salt of 2-thiosulfonate anion which is the coenzyme in methanogenesis by anaerobic archaebacteria.[
As a mucolytic agent, it disrupts disulfide bonds of the mucous polypeptide chains. This feature is used in respiratory medicine (in the treatment of chronic bronchitis, asthma, chronic pharyngolaryngitis, etc.) and also in some surgical treatments.[
By the agency of the knowledge that the physiologic and pathologic adhesions between different layers are rich in disulfide bonds, MESNA has gained attention in recent years as “a promising chemical dissector” in neurotologic surgery to provide a cleavage between tumor and important structures of nervous system such as cranial nerves, brain, cerebellum, brainstem, arteries, and veins.[
MATERIALS AND METHODS
Animals
Fifteen healthy adult male albino guinea pigs were used in this study. Subjects were acclimatized to laboratory conditions for 7 days at animal experiments laboratory. All animal handling was done as prescribed by Declaration of Helsinki and in compliance with Gazi University Animal Experiments Local Ethics Committee regulations (GU-ET 13.056). Subjects were divided into three groups. Fifty percent MESNA was applied to six guinea pigs in study-1 group. In study-2 group, also six subjects were applied 100% MESNA. And three subjects were applied saline only as the control group.
Anesthesia
The animals were anesthetized by 2% of the compound solution of 5 mg/kg xylazine (Rompun®, Bayer Vital, Leverkusen, Germany) and 35 mg/kg ketamin HCl (Ketalar®, Eczacibasi Warner Lambert, Istanbul, Turkey) intramuscular injection.
Surgery
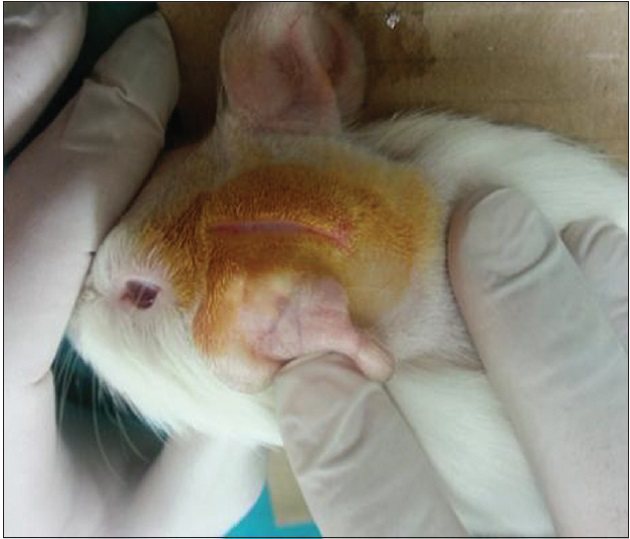
Surgery was performed under a surgical microscope and sterile conditions. A supraauricular 2 cm incision was performed on the left side for all subjects [
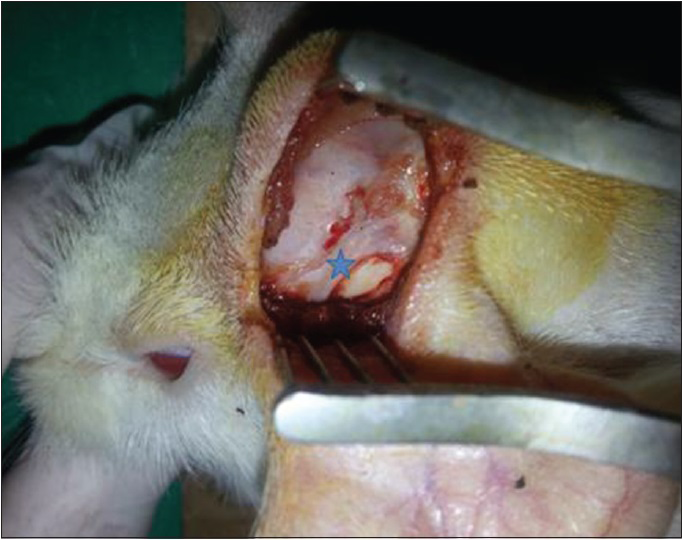
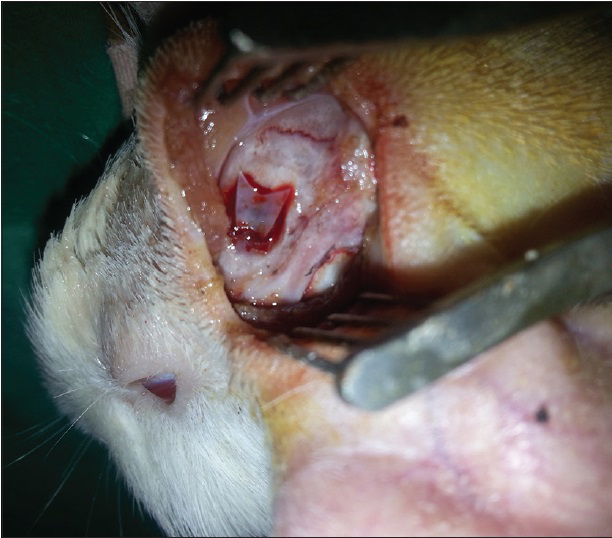
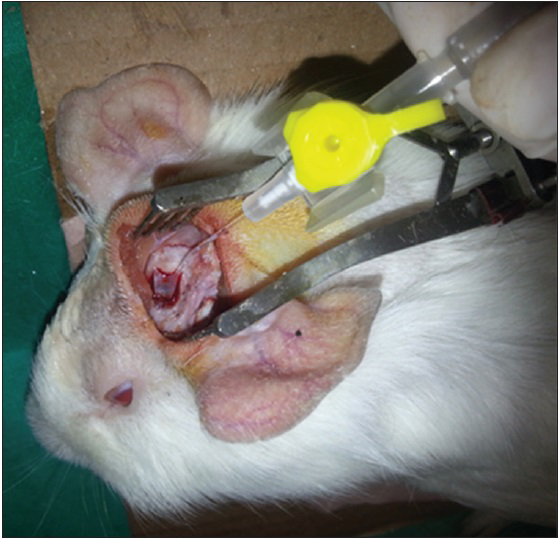
After the dura was opened as a flap, cerebrospinal fluid was released. Penrose drain was put to avoid the injury to the brain tissue and 1–2 cc injection (study-1 group: 50% MESNA and 50% saline compound, study-2 group: 100% MESNA, control group: Saline) was done over the Penrose to the subarachnoid space and waited with 45° elevation of the head for 5 min to delay the escape of the fluid [Figures
Evaluation of the materials
Following the sacrification, the brain tissues were harvested and prepared according to hematoxylin-eosin staining protocol. The microscopic evaluation was carried out to detect whether the following were present in the samples:
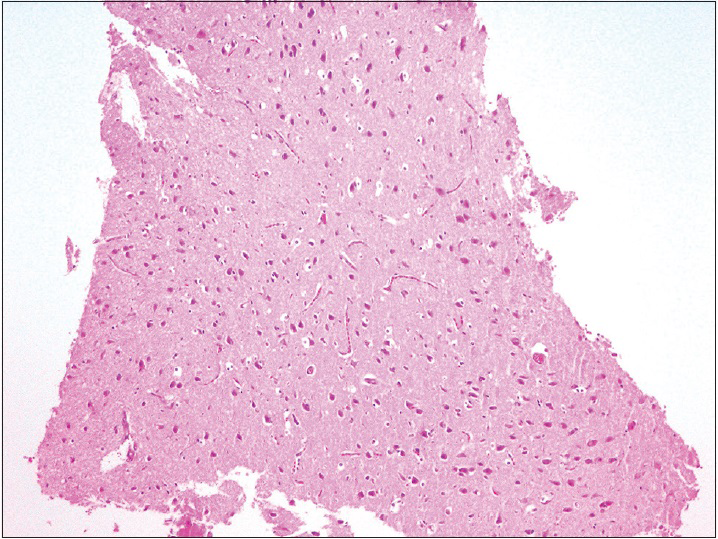
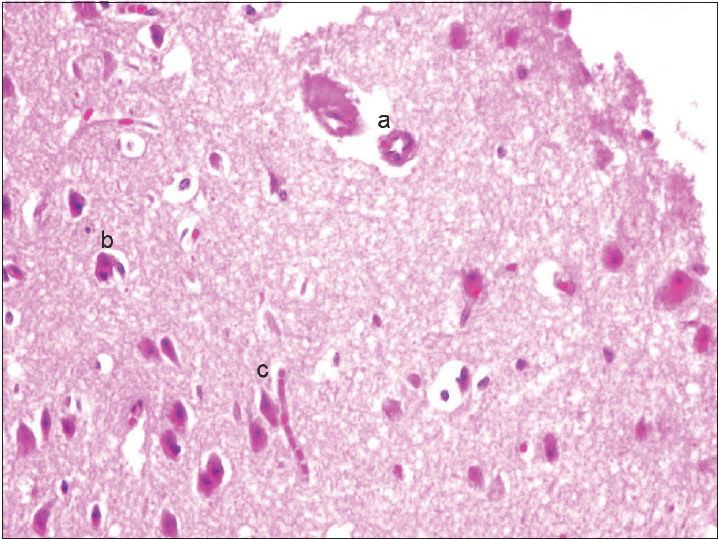
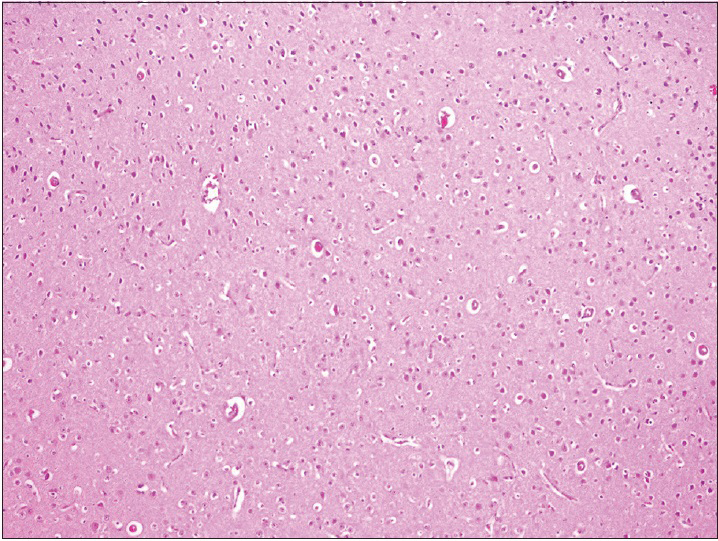
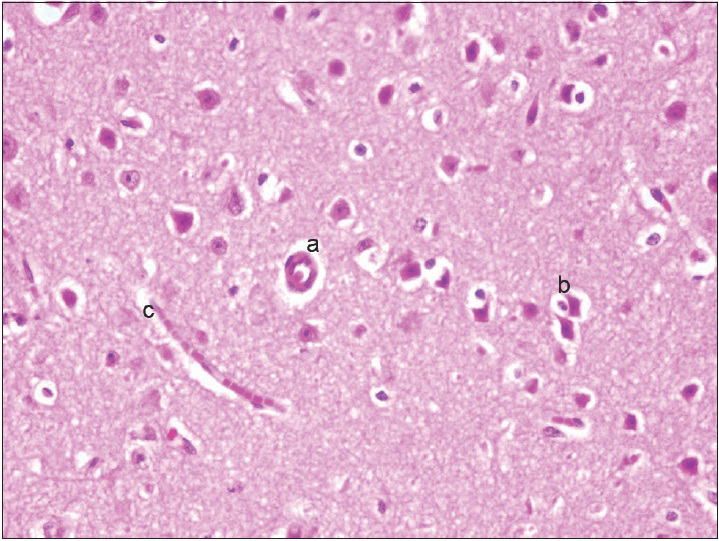
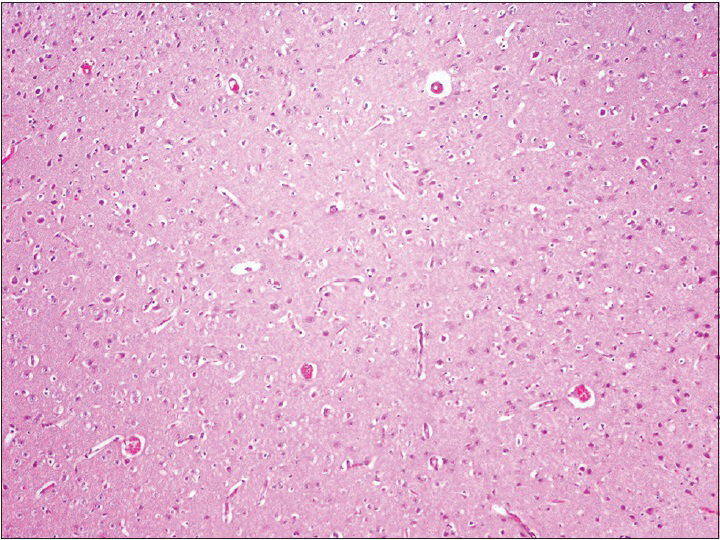
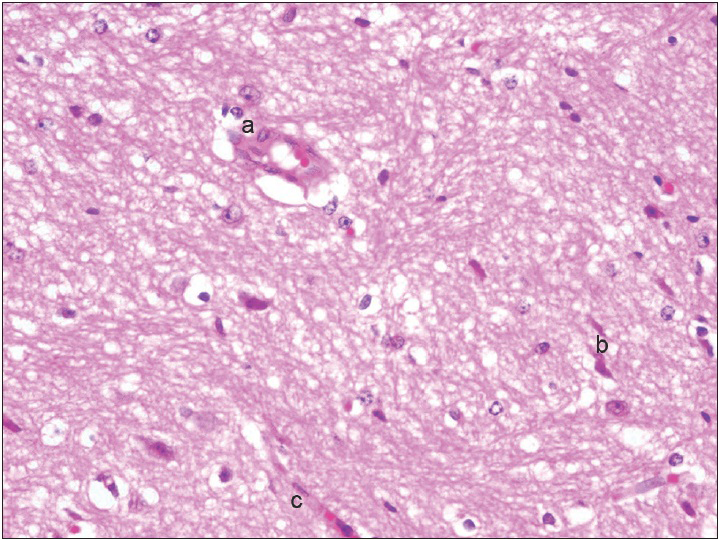
Neurons: Loss, nuclear or cytoplasmic inclusions, evidence of ischemia, loss of normal cytoplasmic volume and organelles such as endoplasmic reticulum, intactness of the nuclear structure, and the presence of nucleoli Astrocytes: Increased number, gemistocytic transformation, evidence of gliosis, nuclear or cytoplasmic enlargement or inclusions Oligodendrogliocytes: Increased number Microglial cells: Presence or predominance of these cells, presence of microglial islands or nodules Blood vessels: Hyperplasia or hypertrophy, evidence of endothelial proliferation, hemorrhage Leptomeningeal tissue: Disruption of the natural lining of these cells, alterations in the blood vessels therein Inflammatory reaction: Presence of lymphocytes, neutrophils, edema.
RESULTS
The observation of the morphological features of brain tissues revealed no alterations in vascular and neural structures with both concentrations of MESNA [Figures
DISCUSSION
Treatment of neural tissue pathologies is a challenging issue because of the high morbidity and mortality rates. In otoneurosurgery, the objective is total tumor extirpation without any life-threatening complication or a loss in quality of life due to inhibitory sequelae. During mechanical dissection of the tumor, brain manipulation can lead to cerebral/cerebellar edema, subdural hematoma or acute brain dysfunction. Cerebrovascular complications, such as carotid artery rupture due to excessive adventitial dissection are also of major concern of the operation. Cranial nerve deficits have an impact on postoperative quality of life and are also potentially life-threatening problems.[
MESNA has been used locally (by irrigation or surgical sponges) in otologic surgery for several years, especially in cases with adhesive otitis media to elevate the adhesive tympanic membrane by intratympanic usage[
As for neurotoxicity, repeated intraperitoneal injection of high dose MESNA (>300 mg/kg) induced spinal activity and contralateral activity of the trigeminoreticular areas of the brainstem-spinal cord junction in rat models.[
Consequently, we predict MESNA as a promising chemical dissector which can be used in neurootologic surgery. But still further information is needed to assess its safety as there are some limitations in our study and also in the former studies. The most important limitation is the method of neural and neurovascular damage assessment. Our study based on morphological analysis of the neural and neurovascular structures after the application of MESNA. Functional neurological assessment, which is the gold standard evaluation method of the neural function is still lacking.
A reduction in morbidity and mortality can be expected by a chemical dissector due to avoiding mechanical trauma to neural and neurovascular tissues during neurootological surgery. However, it is not probable to inform this data as this is a preliminary animal study, comparative trial in humans is still needed. Further studies must interest in neurophysiological assessments in animals and human safety trials.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Casale M, Di Martino A, Salvinelli F, Trombetta M, Denaro V. MESNA for chemically assisted tissue dissection. Expert Opin Investig Drugs. 2010. 19: 699-707
2. Dolgun H, Sekerci Z, Turkoglu E, Kertmen H, Yilmaz ER, Anlar M. Neuroprotective effect of mesna (2-mercaptoethane sulfonate) against spinal cord ischemia/reperfusion injury in rabbits. J Clin Neurosci. 2010. 17: 486-9
3. Dorr RT. Chemoprotectants for cancer chemotherapy. Semin Oncol. 1991. 18: 48-58
4. Flint PW, Haughey BH, Lund VJ.editors. Cummings Otolaryngology: Head and Neck Surgery. Philadelphia, PA: Mosby; 2010. 174: 2468-9
5. Kabasakal L, Sehirli AO, Cetinel S, Cikler E, Gedik N, Sener G. Mesna (2-mercaptoethane sulfonate) prevents ischemia/reperfusion induced renal oxidative damage in rats. Life Sci. 2004. 75: 2329-40
6. Menétrey D, Bon K, Michiels JF, Lantéri-Minet M. The uroprotection of mesna on cyclophosphamide cystitis in rats. Its consequences on behaviour and brain activities. C R Acad Sci III. 1999. 322: 505-15
7. Sener G, Sehirli O, Ercan F, Sirvanci S, Gedik N, Kacmaz A. Protective effect of MESNA (2-mercaptoethane sulfonate) against hepatic ischemia/reperfusion injury in rats. Surg Today. 2005. 35: 575-80
8. Thauer RK. Biochemistry of methanogenesis: A tribute to Marjory Stephenson 1998 Marjory Stephenson prize lecture. Microbiology. 1998. 144: 2377-406
9. van Spaendonck MP, Timmermans JP, Claes J, Scheuermann DW, Wuyts FL, van de Heyning PH. Single ototopical application of mesna has no ototoxic effects on guinea pig cochlear hair cells: A morphological study. Acta Otolaryngol. 1999. 119: 685-9
10. Vincenti V, Mondain M, Pasanisi E, Piazza F, Puel JL, Bacciu S. Cochlear effects of mesna application into the middle ear. Ann N Y Acad Sci. 1999. 884: 425-32
11. Yilmaz ER, Kertmen H, Gürer B, Kanat MA, Arikok AT, Ergüder BI. The protective effect of 2-mercaptoethane sulfonate (MESNA) against traumatic brain injury in rats. Acta Neurochir (Wien). 2013. 155: 141-9
12. Yilmaz M, Goksu N, Bayramoglu I, Bayazit YA. Practical use of MESNA in atelectatic ears and adhesive otitis media. ORL J Otorhinolaryngol Relat Spec. 2006. 68: 195-8
13. Ypsilantis P, Lambropoulou M, Tentes I, Kortsaris A, Papadopoulos N, Simopoulos C. Mesna protects intestinal mucosa from ischemia/reperfusion injury. J Surg Res. 2006. 134: 278-84
14. Zini C, Bacciu S, Gandolfi A, Piazza F, Pasanisi E. Use of Sodium 2-Mercaptoethanesulfonate in Surgery, Patent WO016213. 1998. p.