- Department of Neurosurgery, Princess Alexandra Hospital, Woolloongabba, Australia
- Department of Neurosurgery, Canberra Hospital, Garran, Australia.
- Department of Radiology, Canberra Hospital, Garran, Australia.
- Department of Radiology, Princess Alexandra Hospital, Woolloongabba, Australia.
Correspondence Address:
Alexander Lam, Department of Neurosurgery, Princess Alexandra Hospital, Woolloongabba, Australia.
DOI:10.25259/SNI_208_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Alexander Lam1, Denesh Selvarajah2, Soe San Htike1, Sophia Chan1, Shivendra Lalloo3, Gregory Lock4, Kendal Redmond4, David Leggett4, Peter Mews2. The efficacy of postoperative middle meningeal artery embolization on chronic subdural hematoma – A multicentered randomized controlled trial. 12-May-2023;14:168
How to cite this URL: Alexander Lam1, Denesh Selvarajah2, Soe San Htike1, Sophia Chan1, Shivendra Lalloo3, Gregory Lock4, Kendal Redmond4, David Leggett4, Peter Mews2. The efficacy of postoperative middle meningeal artery embolization on chronic subdural hematoma – A multicentered randomized controlled trial. 12-May-2023;14:168. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12322
Abstract
Background: Middle meningeal artery (MMA) embolization has recently emerged as a potential treatment for chronic subdural hematoma (cSDH). Numerous retrospective studies have suggested that it can potentially reduce the risk of hematoma recurrence following surgical evacuation. We have conducted a randomized controlled trial to investigate the effectiveness of postoperative MMA embolization in reducing recurrence rate, residual hematoma thickness as well as improving functional outcome.
Methods: Patients aged 18 or above were recruited. Following evacuation through burr hole or craniotomy, patients were randomly allocated to undergo either MMA embolization or standard care (monitoring). The primary outcome was symptomatic recurrence requiring redo evacuation. Secondary outcomes include residual hematoma thickness and modified Rankin Scale (mRS) at 6 weeks and 3 months.
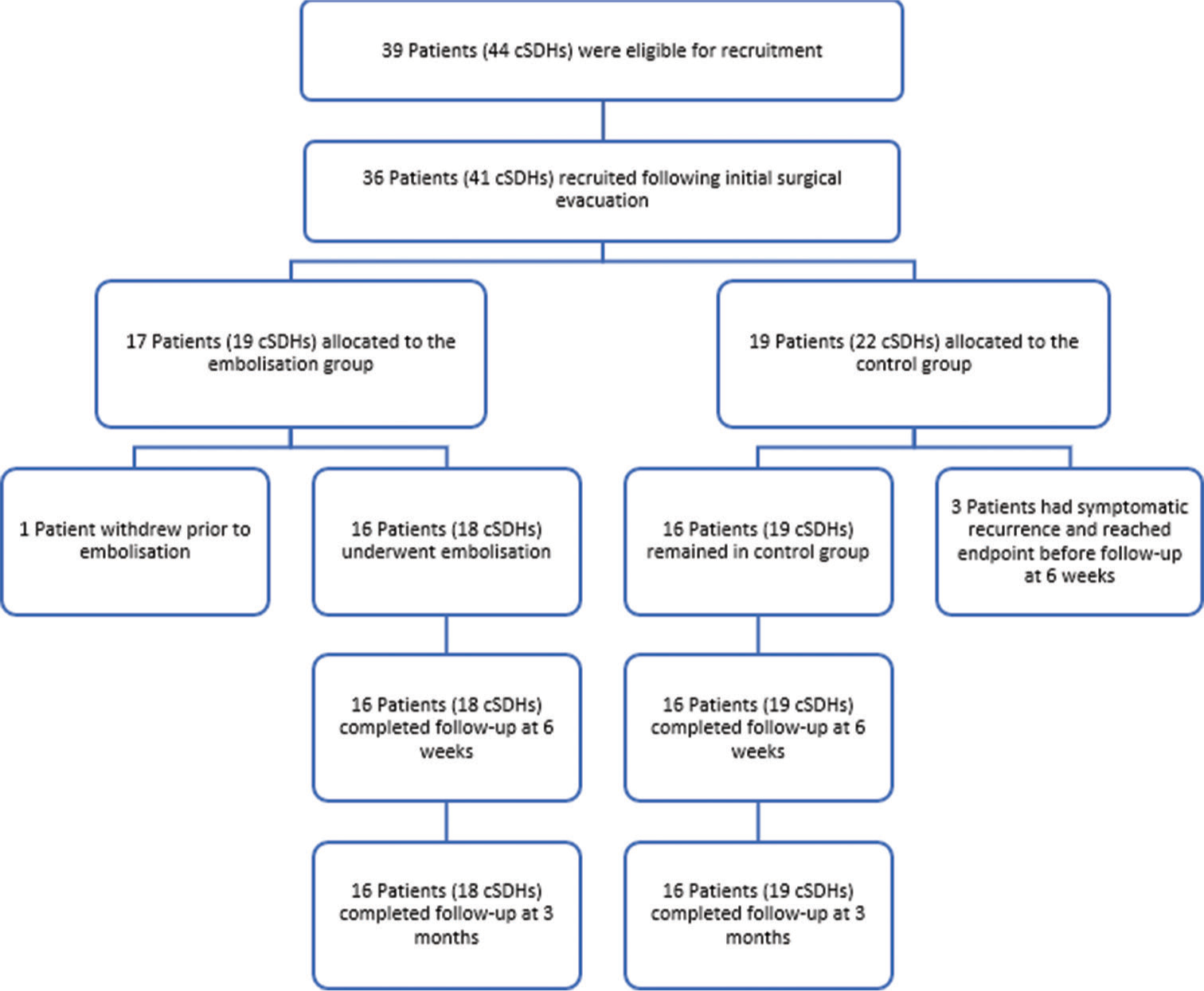
Results: Thirty-six patients (41 cSDHs) were recruited between April 2021 and September 2022. Seventeen patients (19 cSDHs) were allocated to the embolization group and 19 patients (22 cSDHs) were in the control group. No symptomatic recurrence was observed in the treatment group while 3 control patients (15.8%) underwent repeat surgery for symptomatic recurrence, however, it was not statistically significant (P = 0.234). Furthermore, there was no significant difference in residual hematoma thickness at 6 weeks or 3 months between the two groups. All patients in the embolization group had a good functional outcome (mRS 0–1) at 3 months, which was significantly higher than the 53% observed in the control group. No complications related to MMA embolization were reported.
Conclusion: Further study with larger sample size is required to evaluate the efficacy of MMA embolization.
Keywords: Chronic subdural hematoma, Interventional neuroradiology, Middle meningeal artery embolization
INTRODUCTION
Chronic subdural hematoma (cSDH) is a common neurosurgical condition with an estimated incidence of 20.6/100,000 persons/year.[
cSDH is classically believed to be triggered by a minor head injury, which initiates a cascade of inflammatory reactions including dural border cell proliferation, granulation tissue formation, and excessive fibrinolysis.[
Being the main arterial feeder to the subdural inflammatory membranes, the middle meningeal artery (MMA) has been identified as a potential treatment target for cSDH. By embolizing the MMA, it can potentially shift the balance from rebleeding toward reabsorption by reducing the arterial supply to the subdural inflammatory membranes. Numerous case reports and series over the last decade have supported the use of MMA embolization, both as a sole therapy and as an adjunct treatment to surgery.[
In this multicentered randomized controlled trial, we aimed to evaluate the efficacy of postoperative MMA embolization in reducing symptomatic recurrence as well as promoting hematoma reabsorption and improving functional outcomes.
MATERIALS AND METHODS
Study design and patient selection
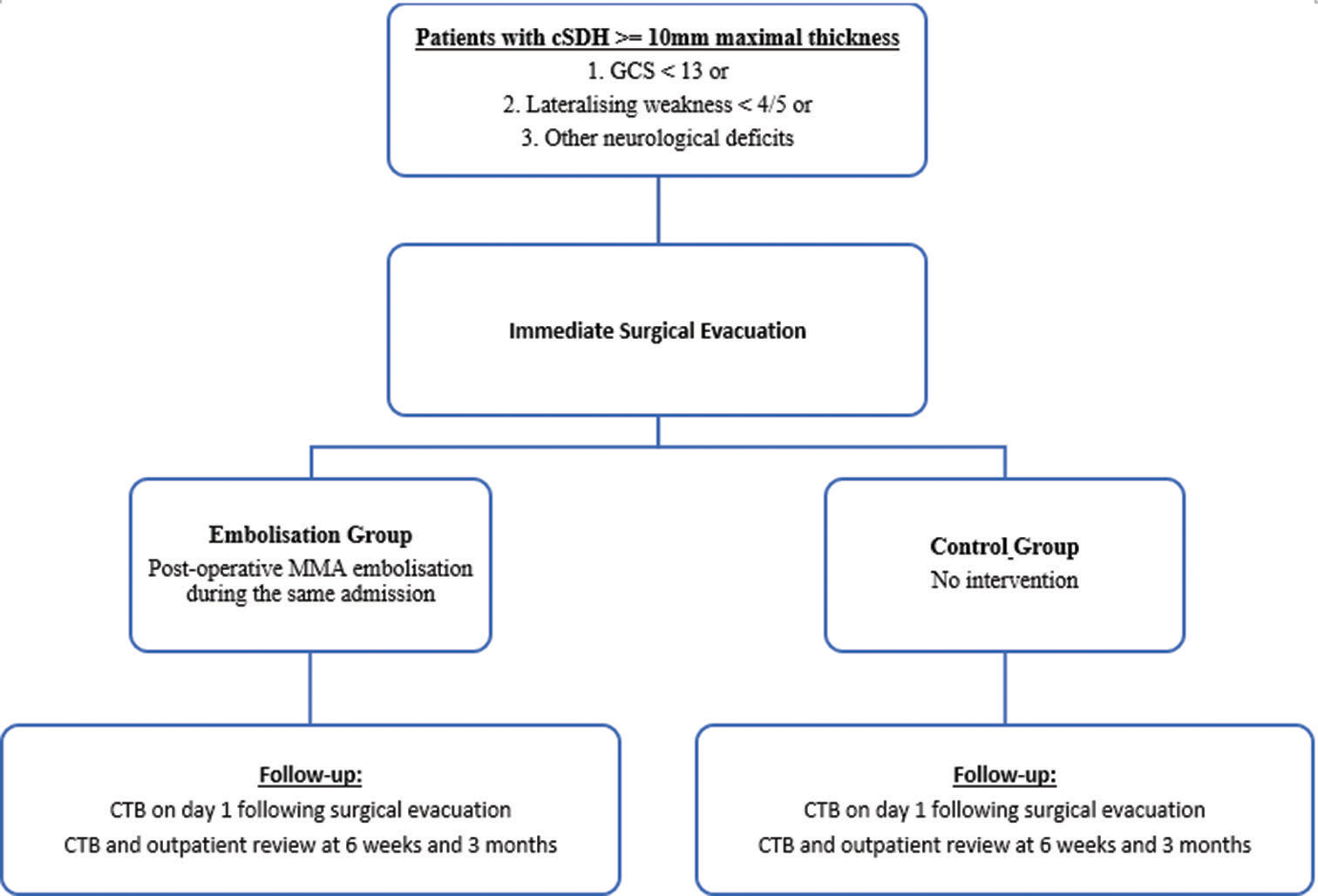
This is a multicentered, nonblinded, randomized controlled trial. It was designed to assess the efficacy of MMA embolization in reducing the recurrence rate of cSDH following initial surgical evacuation. The major null hypothesis was that there would be no difference in the recurrence rate at 3 months between the postoperative embolization group and the control group.
All cSDH patients admitted from April 2021 to September 2022 were screened for recruitment. cSDH diagnosis was confirmed on computed tomography (CT) scans at admission. As illustrated in
The trial was approved by the local Health Human Research Ethics Committee (Reference 2020.ETH.00157, REGIS Reference 2020/ETH01487) and registered on Australian New Zealand Clinical Trials Registry (ACTRN12621000263897p). All participants gave written informed consent. Informed consent would only be obtained from the enduring power of attorney if the participants were incapable of providing consent and had advance health directives with permission to consent for research in place. We followed the National Statement on Ethical Conduct in Human Research in accordance with the Australian National Health and Medical Research Council Act 1992 throughout the study.
Intervention
The embolization procedure was performed by the neurointerventionists. All procedures were performed under general anesthetics following surgical evacuation during the same hospital admission. Systemic heparinization was not used. Femoral or radial access was employed at the discretion of the treating neurointerventionist. Common carotid and external carotid angiography were performed using a standard 5 or 6 French catheter. Under roadmap guidance, a microcatheter (Headway Duo 167, Terumo Microvention) or balloon microcatheter (Scepter XC, Scepter mini, Terumo Microvention) with guidewire was advanced into the MMA. MMA angiography was then performed to identify both frontal and parietal branches as well as to exclude potential dangerous anastomoses. Embolization was performed with one of the following embolic agents including Squid-12 (Balt, Montmorency, France), Onyx-18 (Medtronic, Irvine, CA, USA), Phil 25% (MicroVention, Aliso Viejo, USA), and 25% n-butyl cyanoacrylate (n-BCA) (B. Braun, Melsungen, Germany) with 75% Lipiodol (Guerbet, Villepinte, France), at the discretion of the treating neurointerventionist. When anterograde flow through MMA branches was no longer visible, the procedure was concluded.
Sample size calculation and outcome measures
Based on the current literature,[
The primary outcome was symptomatic recurrence requiring repeat surgical evacuation. Recurrence of cSDH was defined as radiologically persistent or new cSDH with persistent or new symptoms secondary to the mass effect of the cSDH. Secondary outcomes include mRS at 6 weeks and 3 months, the maximal thickness of residual cSDH as measured on CT at 6 weeks and 3 months, as well as all surgical or endovascular complications. A complication was defined as any adverse event related to surgery or embolization. It includes infection, new neurological deficits, seizures, pseudoaneurysms, retroperitoneal hemorrhage, allergic reactions to contrast or medications, stroke, intracerebral hemorrhage, carotid dissection, deep vein thrombosis, pulmonary embolism, myocardial infarction, and death.
Data collection
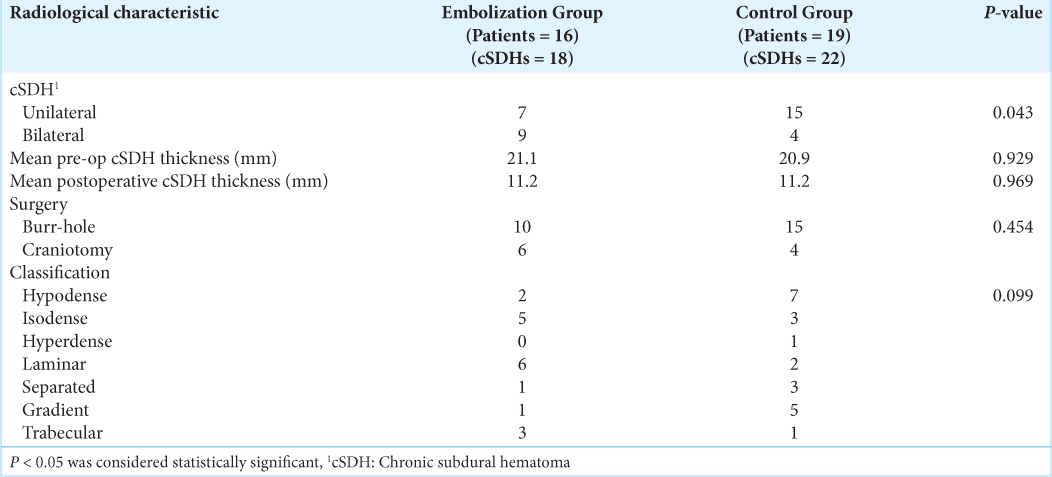
Clinical and radiological data were collected prospectively. The maximal thickness of residual cSDH as measured on CT at 6 weeks and 3 months was reported by independent neuroradiologists unrelated to this study. mRS at 6 weeks and 3 months was assessed during outpatient review by the researchers. All cSDHs were classified according to the imaging appearance as described by Nakaguchi et al. into seven types, which include hypodense, isodense, hyperdense, laminar, separated, gradient, and trabecular.[
Patient safety
Recruitment, blinded outcomes, and adverse events were monitored every 3 months by an independent Data Safety Monitoring Board comprised a consultant radiologist and consultant neurologist not affiliated with the study.
Statistical analyses
Statistical analysis was performed using Stata V17 (StataCorp. 2021. Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC). Statistical significance was set at P < 0.05. Categorical variables were summarized using counts, while continuous variables were summarized using means. Comparisons were made between the embolization and the control group for all relevant variables. Categorical variables were analyzed using Fisher’s exact tests, while continuous variables were analyzed using two-sample t-tests. P < 0.05 indicates a statistically significant difference between the two groups. Due to the small sample size, we were unable to conduct statistical comparisons for certain variables such as complications and mortality. A summary of counts is presented for these variables.
RESULTS
As shown in
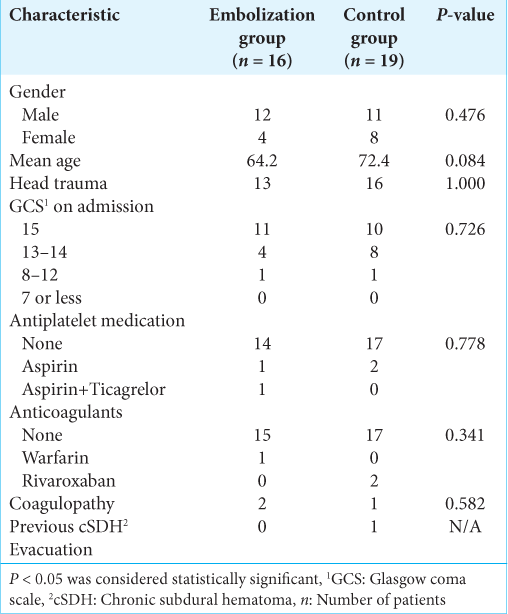
The patient demographic characteristics are summarized in
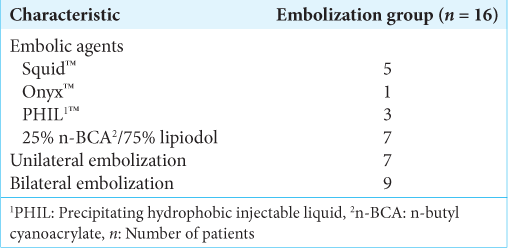
The radiological and treatment characteristics are shown in
As illustrated in
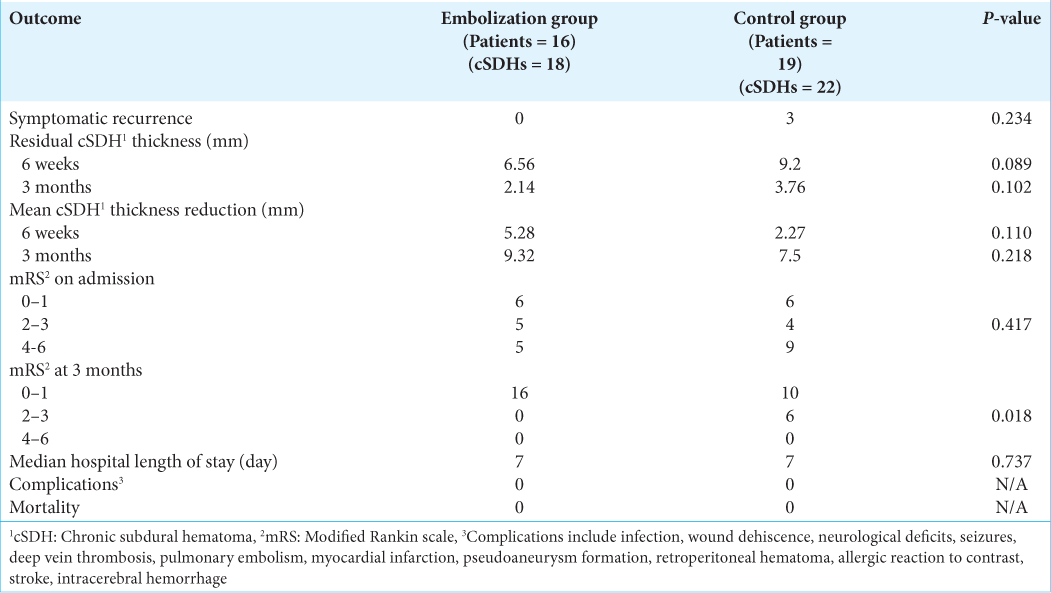
The results of the primary and secondary outcomes are summarized in
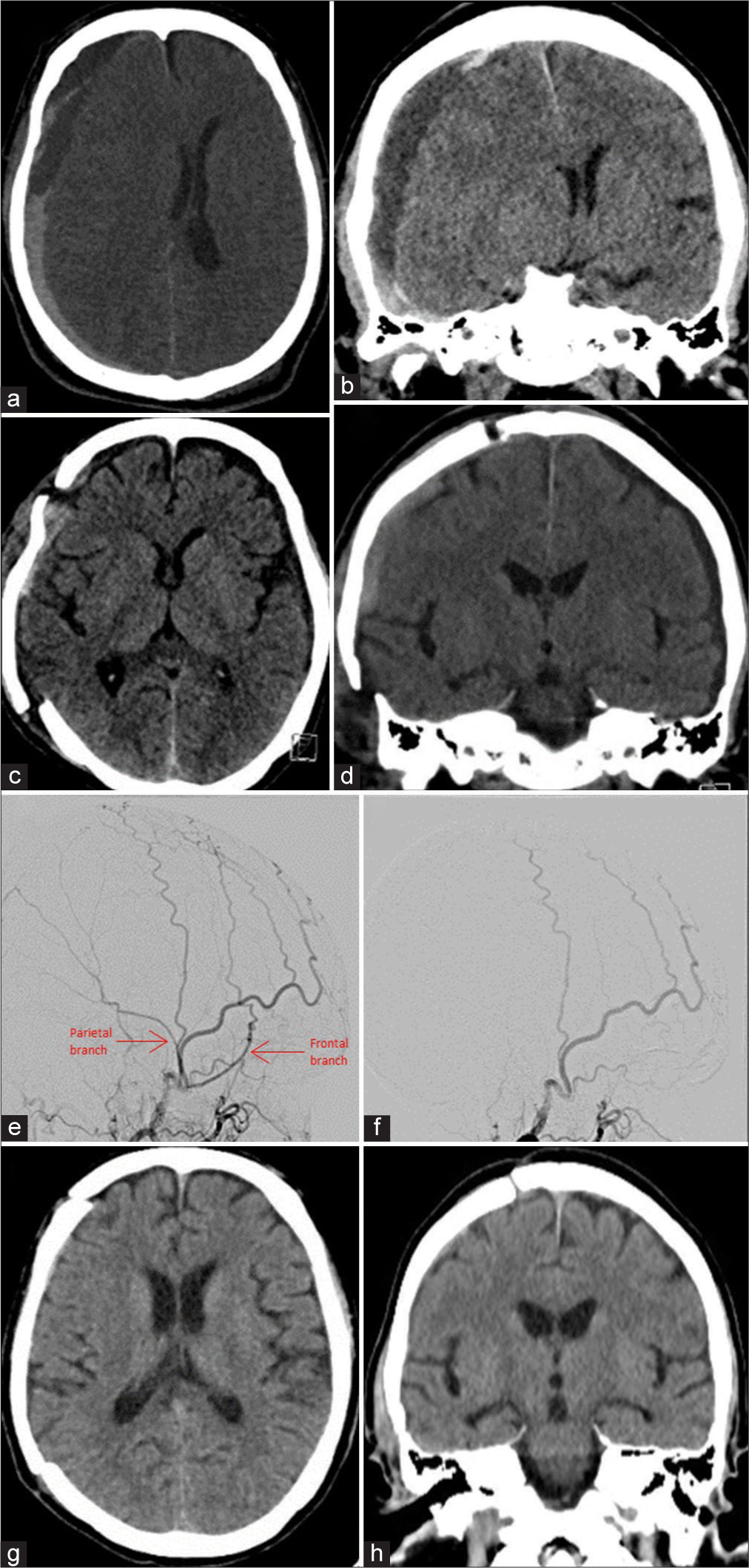
Figure 3:
Example of a participant in the embolization group. (a) Preop axial computed tomography (CT) demonstrating a right convexity chronic subdural hematoma. (b) Pre-op coronal CT. (c) Postopereative axial CT. (d) Postoperative coronal CT. (e) Right middle meningeal artery (MMA) angiogram demonstrating flow in the frontal and parietal branches. (f) Post-embolization angiogram demonstrating occlusion of the right MMA branches. (g) Axial CT at 6 weeks demonstrating complete resolution. (h) Coronal CT at 6 weeks.
DISCUSSION
The results of this study did not demonstrate a significant reduction in recurrence rate following postoperative MMA in patients with cSDH. This is similar to the findings of the randomized controlled trial by Ng et al. 2020.[
Our results also did not show a difference in residual hematoma thickness at 6 weeks and 3 months between the embolization group and the control group. Ng et al. 2020 used cSDH volume instead of maximal thickness in their study and showed that postoperative MMA embolization was associated with a higher volume of hematoma resorption at 3 months when compared to the control group (52.6 mL vs. 35.1 mL). Even though hematoma volume is potentially a more sensitive way in detecting any subtle changes in hematoma size, we believe that maximal thickness carries more clinical relevance as it directly reflects the local mass effect.
Finally, a significantly higher proportion of patients with good functional outcomes (mRS of 0 or 1) was seen in the embolization group when compared to the control group. Headache was the most common symptom reported among patients in both groups and it could partially be related to dural irritation secondary to the inflammatory responses within the hematoma. By limiting its vascular supply to the chronic inflammatory membranes, MMA embolization might be able to reduce such inflammatory responses and therefore dural irritation.
The major limitation of this study is that it is underpowered. According to the current literature, the recurrence rate of cSDH following surgical evacuation was ranging widely from 10% to 37%.[
One criticism leveled at this study is that the embolization procedure was not standardized in the current study; neither was the surgical technique. Various embolic agents were used depending on the discretion of the treating neurointerventionist. This was a conscious decision in the design of the study, which aimed to demonstrate the efficacy of the treatment endpoint (embolization of the MMA) and not a specific embolic agent. Furthermore, patients at TCH received MMA embolization that was ipsilateral to the side of the cSDH and bilateral embolization only in case of bilateral cSDHs. On the other hand, all patients at PAH received bilateral embolization regardless of the laterality of the cSDH. Nevertheless, there is insufficient evidence from the literature to suggest the superiority of a particular embolic agent over the others or whether bilateral embolization would be more effective than unilateral embolization for patients with unilateral cSDH. Further studies are required to answer these questions.
CONCLUSION
Our interim results have demonstrated MMA embolization as having a potential role to serve as a postoperative adjunct treatment for cSDH. We demonstrated that postoperative MMA embolization is associated with an improved functional outcome at 3 months. However, an ongoing study with a larger sample size will be required to confirm its efficacy in reducing the recurrence rate as well as facilitating the resolution of residual hematoma. Once its efficacy is confirmed, it would be a particularly useful treatment for patients with recurrent cSDH or those with the significant risk associated with prolonged cessation of anticoagulation therapy.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Life Health Care Group.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Almenawer SA, Farrokhyar F, Hong C, Alhazzani W, Manoranjan B, Yarascavitch B. Chronic subdural hematoma management: A systematic review and meta-analysis of 34,829 patients. Ann Surg. 2014. 259: 449-57
2. Balser D, Farooq S, Mehmood T, Reyes M, Samadani U. Actual and projected incidence rates for chronic subdural hematomas in United States Veterans Administration and civilian populations. J Neurosurg. 2015. 123: 1209-15
3. Ban SP, Hwang G, Byoun HS, Kim T, Lee SU, Bang JS. Middle meningeal artery embolization for chronic subdural hematoma. Radiology. 2018. 286: 992-9
4. Hashimoto T, Ohashi T, Watanabe D, Koyama S, Namatame H, Izawa H. Usefulness of embolization of the middle meningeal artery for refractory chronic subdural hematomas. Surg Neurol Int. 2013. 4: 104
5. Kang J, Whang K, Hong SK, Pyen JS, Cho SM, Kim JY. Middle meningeal artery embolization in recurrent chronic subdural hematoma combined with arachnoid cyst. Korean J Neurotrauma. 2015. 11: 187-90
6. Karibe H, Kameyama M, Kawase M, Hirano T, Kawaguchi T, Tominaga T. Epidemiology of chronic subdural hematomas. No Shinkei Geka. 2011. 39: 1149-53
7. Kim E. Embolization therapy for refractory hemorrhage in patients with chronic subdural hematomas. World Neurosurg. 2017. 101: 520-7
8. Link TW, Boddu S, Marcus J, Rapoport BI, Lavi E, Knopman J. Middle meningeal artery embolization as treatment for chronic subdural hematoma: A case series. Oper Neurosurg (Hagerstown). 2018. 14: 556-62
9. Link TW, Boddu S, Paine SM, Kamel H, Knopman J. Middle meningeal artery embolization for chronic subdural hematoma: A series of 60 cases. Neurosurgery. 2019. 85: 801-7
10. Link TW, Rapoport BI, Paine SM, Kamel H, Knopman J. Middle meningeal artery embolization for chronic subdural hematoma: Endovascular technique and radiographic findings. Interv Neuroradiol. 2018. 24: 455-62
11. Link TW, Schwarz JT, Paine SM, Kamel H, Knopman J. Middle meningeal artery embolization for recurrent chronic subdural hematoma: A case series. World Neurosurg. 2018. 118: e570-4
12. Matsumoto H, Hanayama H, Okada T, Sakurai Y, Minam H, Masuda A. Which surgical procedure is effective for refractory chronic subdural hematoma? Analysis of our surgical procedures and literature review. J Clin Neurosci. 2018. 49: 40-7
13. Nakaguchi H, Tanishima T, Yoshimasu N. Factors in the natural history of chronic subdural hematomas that influence their postoperative recurrence. J Neurosurg. 2001. 95: 256-62
14. Ng S, Derraz I, Boetto J, Dargazanli C, Poulen G, Gascou G. Middle meningeal artery embolization as an adjuvant treatment to surgery for symptomatic chronic subdural hematoma: A pilot study assessing hematoma volume resorption. J Neurointerv Surg. 2020. 12: 695-9
15. Stanisic M, Aasen AO, Pripp AH, Lindegaard KF, RammPettersen J, Lyngstadaas SP. Local and systemic pro-inflammatory and anti-inflammatory cytokine patterns in patients with chronic subdural hematoma: A prospective study. Inflamm Res. 2012. 61: 845-52
16. Weigel R, Schmiedek P, Krauss JK. Outcome of contemporary surgery for chronic subdural haematoma: Evidence based review. J Neurol Neurosurg Psychiatry. 2003. 74: 937-43
17. Yagnik KJ, Goyal A, Van Gompel JJ. Twist drill craniostomy vs burr hole drainage of chronic subdural hematoma: A systematic review and meta-analysis. Acta Neurochir (Wien). 2021. 163: 3229-41