- Department of Neurosurgery, University Hospital of Alexandroupolis, Medical School of Democritus University of Thrace, Dragana, Alexandroupolis, Greece,
- Department of Neurosurgery, Universitätsmedizin Göttingen, Georg-August-Universität Göttingen, Göttingen, Germany.
Correspondence Address:
Ntenis Nerntengian, Department of Neurosurgery, University Hospital of Alexandroupolis, Medical School of Democritus University of Thrace, Dragana, Alexandroupolis, Greece.
DOI:10.25259/SNI_158_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ntenis Nerntengian1, Levent Tanrikulu2, Michael Anthony Manoussos1, Nikolaos Barettas1, Grigorios Gkasdaris1, Theodosios Birbilis1. The evaluation of the usefulness of CO2 laser in microsurgical resection of brain tumors. 31-Mar-2022;13:118
How to cite this URL: Ntenis Nerntengian1, Levent Tanrikulu2, Michael Anthony Manoussos1, Nikolaos Barettas1, Grigorios Gkasdaris1, Theodosios Birbilis1. The evaluation of the usefulness of CO2 laser in microsurgical resection of brain tumors. 31-Mar-2022;13:118. Available from: https://surgicalneurologyint.com/surgicalint-articles/11500/
Abstract
Background: Since its introduction to surgery, the CO2 laser has been used in the treatment of various neurosurgical pathologies as it combines cutting, vaporizing, and coagulating properties in one tool and has a safe penetration depth. In this case series of 29 patients, we present the evaluation of the usefulness of the closed system type – sealed tube surgical CO2 laser in the surgical removal of brain tumors.
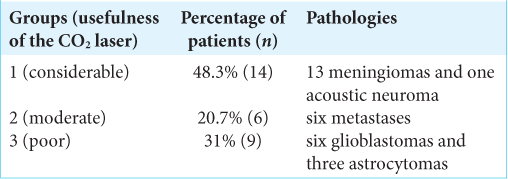
Methods: The Sharplan 40C model SurgiTouch, sealed tube type CO2 laser, was used in the resection of 29 brain tumors; 13 meningiomas, six metastases, nine gliomas, and one acoustic neuroma. The same senior surgeon (BT) assessed and classified the benefit provided by the CO2 laser in the resection of the neoplasms to considerable (Group 1), moderate (Group 2), and poor (Group 3).
Results: Group 1 included 14 patients with 13 meningiomas and one acoustic neuroma, Group 2 included six patients, all of whom had metastases, and Group 3 included nine patients of which six had glioblastoma and three astrocytoma. No complications or technical problems occurred due to the use of the CO2 laser.
Conclusion: The CO2 laser is a valuable complementary tool in brain tumor surgery displaying high efficacy and practicality in the resection of neoplasms which are fibrous and have hard consistency. It has high acquisition and maintenance cost and cannot replace the bipolar diathermy. The newest generation of flexible CO2 laser fiber provides more ergonomy and promises new perspectives of its neurosurgical use in the modern era.
Keywords: Brain tumor surgery, CO2 laser, Laser neurosurgery, Laser surgery, Sealed tube surgical CO2 laser
INTRODUCTION
The term LASER is an acronym for light amplification by stimulated emission of radiation.[
MATERIALS AND METHODS
Technical description of the CO2 laser device
In the neurosurgical department of the Democritus University of Thrace, the CO2 laser sealed tube type was used during surgery of the here reported brain neoplasms. It is a Sharplan 40C model SurgiTouch MS 780 Flash scan 40 Watt, with a wavelength of 10.6 μm and energy output of 750 mJ [
Patient characteristics and usefulness criteria
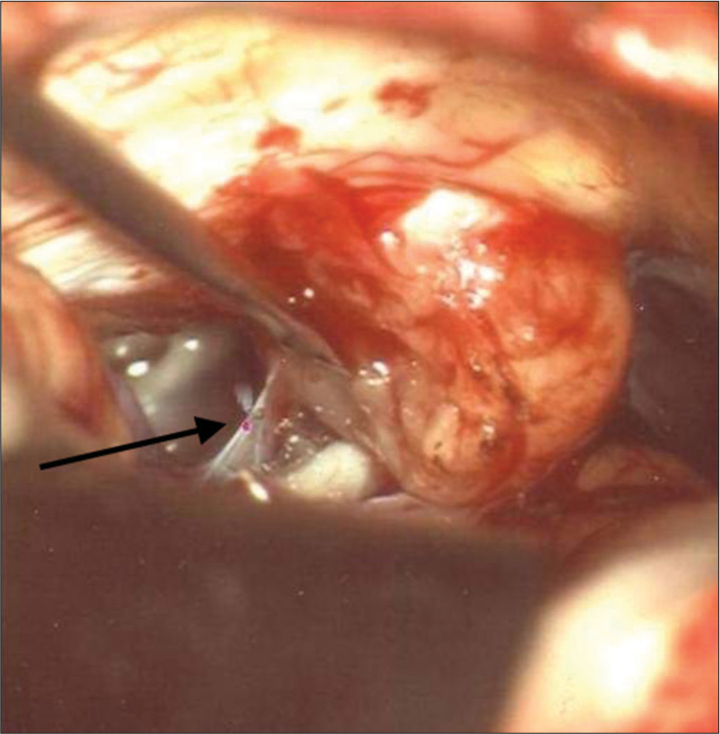
Twenty-nine patients with brain tumors were treated with the use of the CO2 laser between 2009 and 2011. Tumors operated with the conventional technique in this time period (bipolar, ultrasonic aspirator, and microsurgical instruments) were not included in this study. The patients had various types of neoplasms of the brain and are represented in [
RESULTS
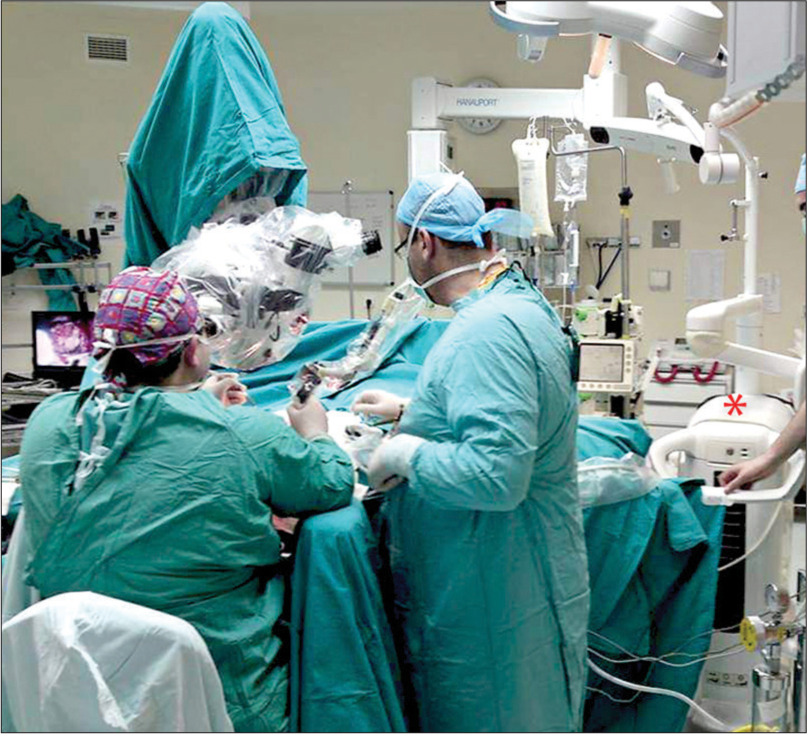
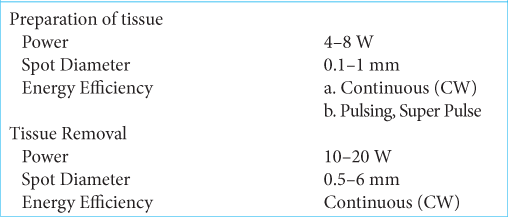
In 26 (90%) of the patients, the joystick was used (f = 125–200 mm); and for three patients, the micromanipulator (f = 200–400 mm) was used. For the preparation and removal of tissues, the CO2 laser was used according to the parameters as described in [
DISCUSSION
The CO2 laser emits an invisible beam of radiation in the field of infrared light, with a wavelength of 10.6 μm, which is absorbed to a great degree by molecules of water[
CONCLUSION
The CO2 laser is a versatile tool that combines the abilities of tissue vaporization, cutting and in a lesser degree of coagulation with minimal thermal and no mechanical trauma to the surrounding tissue. Its uttermost advantage in brain tumor surgery is exhibited in resection of brain tumors with fibrous and hard consistency. The high acquisition and maintenance cost may restrict its use in resource-limited settings and cannot replace bipolar diathermy. The newest generation of flexible CO2 laser fiber provides more ergonomy and unlocks new perspectives of its utilization in neurosurgery.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors thank Mrs. Sophia Coelho for her technical support in the editing of the images.
References
1. Allemann IB, Kaufman J. Laser principles. Curr Probl Dermatol. 2011. 42: 7-23
2. Andersen K. Laser technology--a surgical tool of the past, present, and future. AORN J. 2003. 78: 794-802
3. Cerullo LJ, Burke LP. Use of the laser in neurosurgery. Surg Clin North Am. 1984. 64: 995-1000
4. Choudhri O, Lober RM, Camara-Quintana J, Yeom KW, Guzman R, Edwards MS. Carbon dioxide laser for corpus callosotomy in the paediatric population. J Neurosurg Pediatr. 2015. 15: 321-7
5. Consiglieri GD, Killory BD, Germain RS, Spetzler RF. Utility of the CO2 laser in the microsurgical resection of cavernous malformations. World Neurosurg. 2013. 79: 714-8
6. Deruty R, Pelissou-Guyotat I, Mottolese C, Amat D. Routine use of the CO2 laser technique for resection of cerebral tumours. Acta Neurochir (Wien). 1993. 123: 43-5
7. Devaux BC, Joly LM, Page P, Nataf F, Turak B, Beuvon F. Laser-assisted endoscopic third ventriculostomy for obstructive hydrocephalus: technique and results in a series of 40 consecutive cases. Lasers Surg Med. 2004. 34: 368-78
8. Einstein A. Zur Quantentheorie der Strahlung [On the Quantum Theory of Radiation]. Phys Z. 1917. 18: 121-8
9. Gamache FW, Morgello S. The histopathological effects of the CO2 versus the KTP laser on the brain and spinal cord: A canine model. Neurosurgery. 1993. 32: 100-4
10. Graudenz K, Raulin C. Von einsteins quantentheorie zur modernen lasertherapie. Historie des lasers in der dermatologie und ästhetischen medizin [From Einstein’s quantum theory to modern laser therapy. The history of lasers in dermatology and aesthetic medicine]. Hautarzt. 2003. 54: 575-82
11. Jain KK. Lasers in neurosurgery: A review. Lasers Surg Med. 1983. 2: 217-30
12. Killory BD, Chang SW, Wait SD, Spetzler RF. Use of flexible hollow-core CO2 laser in microsurgical resection of CNS lesions: Early surgical experience. Neurosurgery. 2010. 66: 1187-92
13. Koivukangas J, Heikkinen ER. Preliminary experiences with CO2laser and ultrasonic aspirator in brain surgery. Ann Clin Res. 1986. 18: 85-95
14. Lanzafame RJ. Laser safety programs in general surgery. J Laser Appl. 1994. 6: 111-4
15. Lovrić D, Negovetić L, Lupret V, Vidović D, Gnjidić Z. Evaluacija primjene CO2lasera na 134 bolesnika s ekspanzivnim procesima CNS-a [Evaluation of the use of the CO2 laser in 134 patients with expansive processes in the central nervous system]. Acta Chir Iugosl. 1989. 36: 27-43
16. Marks AJ, Teichman JM. Lasers in clinical urology: State of the art and new horizons. World J Urol. 2007. 25: 227-33
17. Massad M, LoCicero J, Matano J, Oba J, Greene R, Gilbert J. Endoscopic thoracic sympathectomy: evaluation of pulsatile laser, non-pulsatile laser, and radiofrequency-generated thermocoagulation. Lasers Surg Med. 1991. 11: 18-25
18. Oh H, Kim J.editors. Clinical application of CO2 laser. CO2 Laser - Optimisation and Application. London, UK: IntechOpen; 2012. p.
19. Oros-Peusquens AM, Loução R, Abbas Z, Gras V, Zimmermann M, Shah NJ. A single-scan, rapid whole-brain protocol for quantitative water content mapping with neurobiological implications. Front Neurol. 2019. 10: 1333
20. Palanker D. Evolution of concepts and technologies in ophthalmic laser therapy. Annu Rev Vis Sci. 2016. 2: 295-319
21. Patel CK. High power carbon dioxide lasers. Sci Am. 1968. 219: 22-3
22. Rebeiz EE. Lasers in otorhinolaryngology-head and neck surgery. J Med Liban. 1994. 42: 242-9
23. Ryan RW, Wolf T, Spetzler RF, Coons SW, Fink Y, Preul MC. Application of a flexible CO(2) laser fibre for neurosurgery: Laser-tissue interactions. J Neurosurg. 2010. 112: 434-43
24. Saleh B, Al-Amri M, El-Gomati M, Zubairy M.editors. The laser. Optics in Our Time. Cham: Springer; 2016. p.
25. Verschueren RC, Koudstaal J, Oldhoff J. The carbon dioxide laser; some possibilities in surgery. Acta Chir Belg. 1975. 74: 197-204
26. Verschueren RJ, Oldhoff J. The carbon-dioxide laser. A new surgical tool. Arch Chir Neerl. 1975. 27: 199-207
27. Zhong H, Wang Z, Yang Z, Zhao F, Wang B, Liu P. CO2 laser soldering for the reconstruction of dural defects in the minipig model. Turk Neurosurg. 2016. 26: 240-5