- Comprehensive Epilepsy Center, Seirei Hamamatsu General Hospital, Hamamatsu, Shizuoka, Japan.
DOI:10.25259/SNI_241_2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Yosuke Masuda, Ayataka Fujimoto, Mitsuyo Nishimura, Keishiro Sato, Hideo Enoki, Tohru Okanishi. The fence post depth electrode technique to control both brain tumors and epileptic seizures in patients with brain tumor-related epilepsy. 27-Sep-2019;10:187
How to cite this URL: Yosuke Masuda, Ayataka Fujimoto, Mitsuyo Nishimura, Keishiro Sato, Hideo Enoki, Tohru Okanishi. The fence post depth electrode technique to control both brain tumors and epileptic seizures in patients with brain tumor-related epilepsy. 27-Sep-2019;10:187. Available from: http://surgicalneurologyint.com/surgicalint-articles/9676/
Abstract
Background: To control brain tumor-related epilepsy (BTRE), both epileptological and neuro-oncological approaches are required. We hypothesized that using depth electrodes (DEs) as fence post catheters, we could detect the area of epileptic seizure onset and achieve both brain tumor removal and epileptic seizure control.
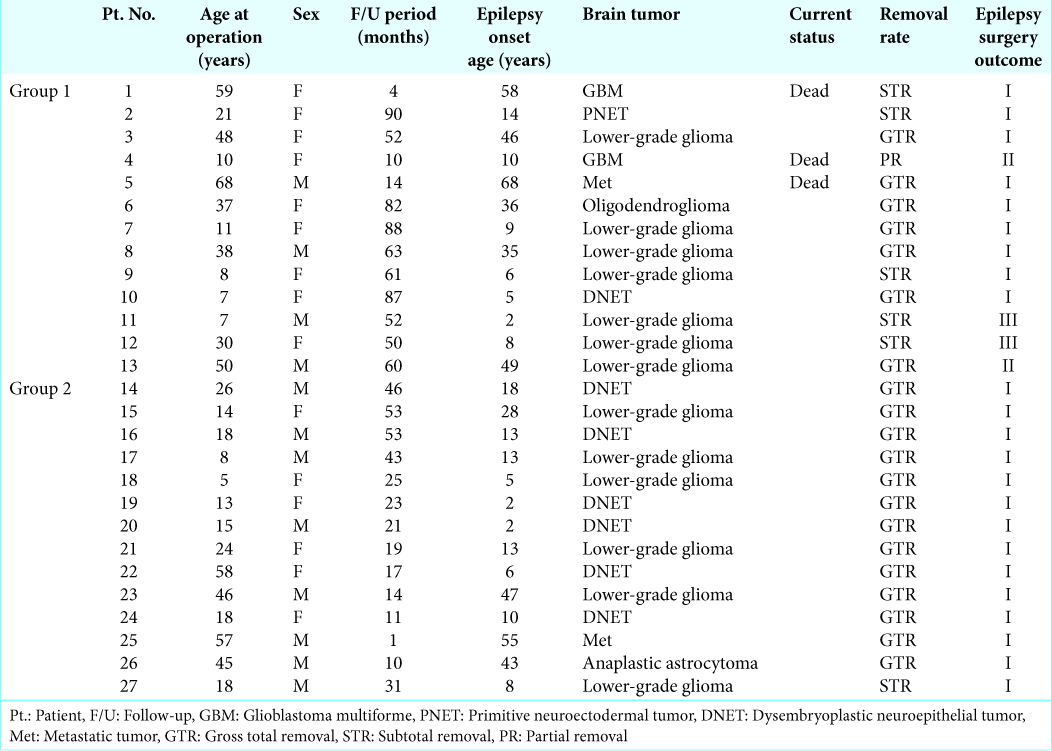
Methods: Between August 2009 and April 2018, we performed brain tumor removal for 27 patients with BTRE. Patients who underwent lesionectomy without DEs were classified into Group 1 (13 patients) and patients who underwent the fence post DE technique were classified into Group 2 (14 patients).
Results: The patients were 15 women and 12 men (mean age, 28.1 years; median age 21 years; range, 5–68 years). The brain tumor was resected to a greater extent in Group 2 than Group 1 (P P P = 0.041).
Conclusion: Using DEs as fence post catheters, we detected the area of epileptic seizure onset and controlled epileptic seizures. Simultaneously, we removed the brain tumor to a greater extent with fence post DEs than without.
Keywords: Brain tumor, Fence post depth electrode technique, Invasive monitoring, Medically refractory epilepsy, Neuronavigation guided
INTRODUCTION
Brain tumor-related epilepsy
Epileptic seizures are one of the most common comorbidities in patients with brain tumors.[
Brain tumors and the fence post catheter technique
Many reports have shown that radical resection is required to control brain tumors.[
Hypothesis and purpose of this study
We hypothesized that using depth electrodes (DEs) as fence post catheters, we could detect the area of epileptic seizure onset and achieve both removal of tumors and control of epileptic seizures in patients with BTRE. In this study, we considered patients who underwent tumor removal surgery without fence post DEs as the control group and compared the outcomes in the control group to those in patients in whom fence post DEs were used.
METHODS
Patients
Between August 2009 and April 2018, we performed brain tumor removal surgery in 47 patients. The patient inclusion criteria in this study were as follows: (1) development of medically refractory epilepsy, (2) surgery was performed by the same surgeon (AF), and (3) follow-up for ≥6 months. Patients who had brain arteriovenous malformation, brain cavernous angioma, subependymal giant cell astrocytoma of tuberous sclerosis complex, or patients who underwent tumor removal without DEs first and then underwent the fence post DE technique for residual seizures during the follow-up period were excluded from the study.
Among these, 27 patients with BTRE met the criteria. We introduced the fence post technique for BTRE in our hospital in August 2013. Thus, patients who underwent lesionectomy without DEs were classified into Group 1 (13 patients; all treated before August 2013). Patients who underwent the fence post DE technique were classified into Group 2 (14 patients; all treated after August 2013).
All patients had undergone presurgical evaluation, including a detailed clinical history and magnetic resonance imaging (MRI).
Clinical information
We reviewed sex, age at surgery, follow-up period, age at epileptic seizure onset, postoperative brain tumor prognosis, location of the brain tumor, the type of brain tumor, and brain tumor removal rate (we defined the removal rate of the brain tumor as GTR: ≥100%; subtotal removal (STR) 90%– <100%; and partial removal (PR) <90%), which DE contacts suggested the seizure onset area, subsequent epilepsy surgery outcome, and complications associated with the fence post DE technique. We evaluated the outcomes of surgery at the end of the follow-up period based on Engel classifications I– IV (Engel I: free of disabling seizures; Engel II: rare disabling seizures; Engel III: worthwhile improvement; and Engel IV: no worthwhile improvement).[
Fence post DE technique
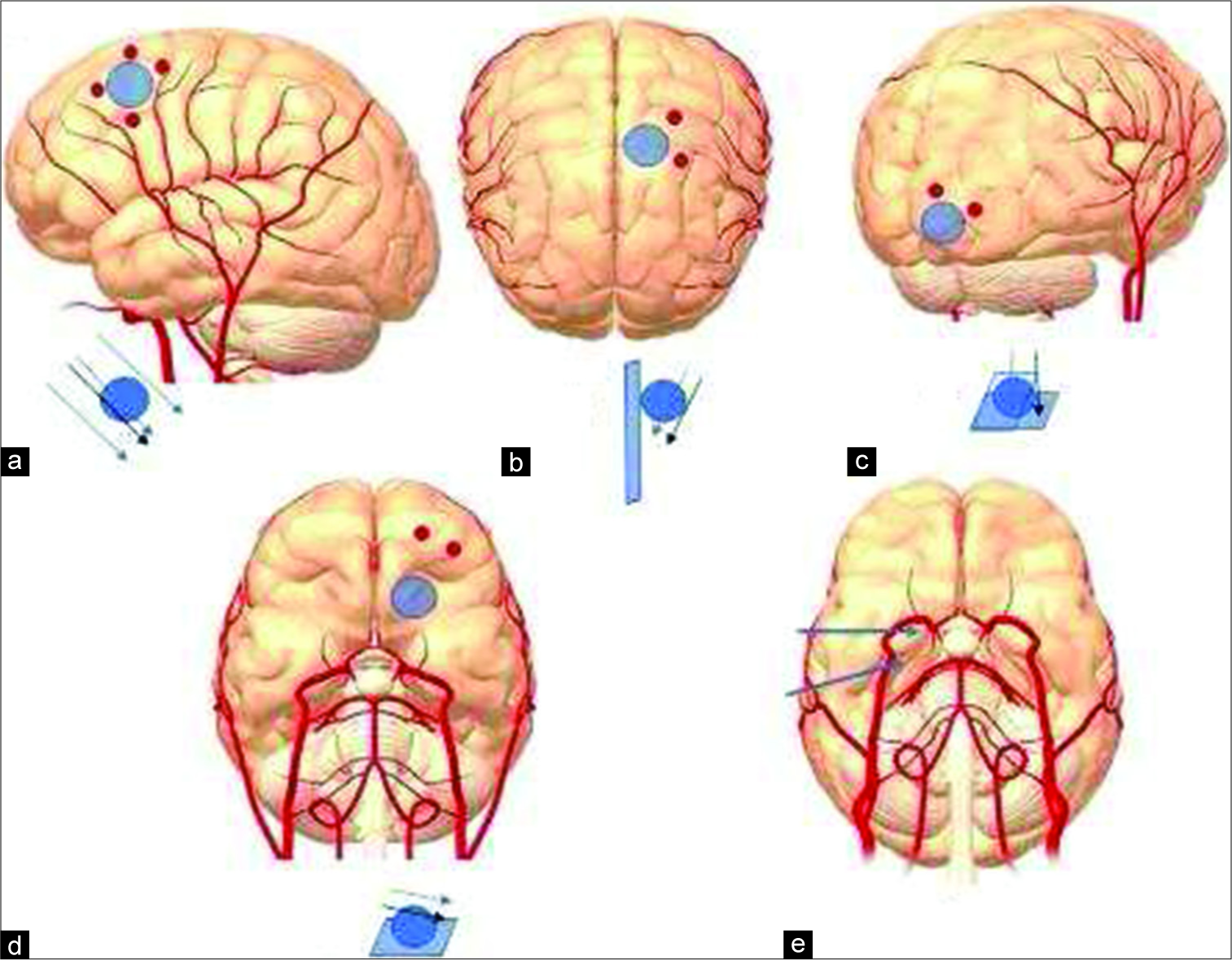
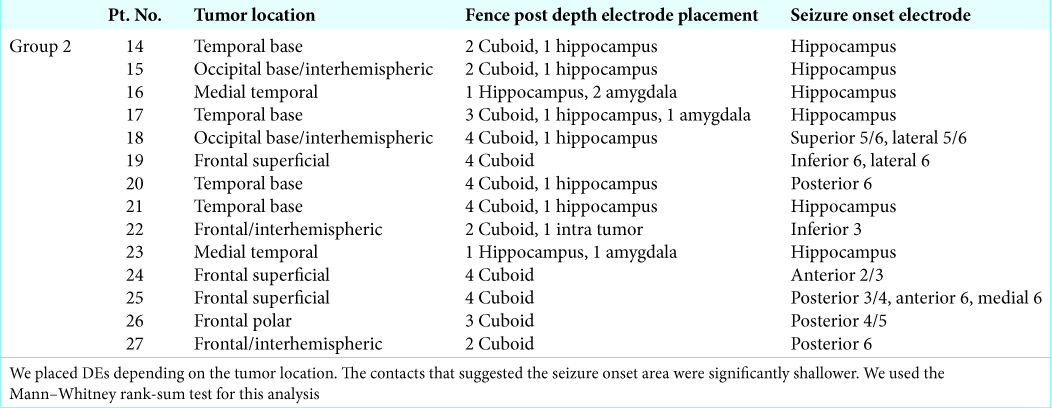
For Group 1, we only used a neuronavigation system to find the tumor location during the operation without using the fence post technique. For Group 2, we used the neuronavigation-guided fence post DE technique. To avoid locational error due to brain shift associated with the neuronavigation system and to allow precise resection of the tumor, we placed the DEs as fence post catheters. A schematic of the fence post procedure is shown in If the tumor was in a superficial area [ If the tumor was in a medial (interhemispheric) area [ If the tumor was in a lateral (polar) area [ If the tumor was in the skull base area [ If the tumor was in an area involving the hippocampus or amygdala [ If the tumor was in an avascular area judging from MRI and/or an angiogram, we inserted the DEs directly into the inside of the tumor.
Figure 1:
Fence post depth electrode placements. (a) A superficial area tumor. We placed three to four depth electrodes (DEs) to contain the tumor within the cuboid. (b) A medial (interhemispheric) area tumor. We placed two to three DEs to make one side of the cuboid. The other side of the cuboid was already on the interhemispheric side. (c) A lateral (polar) area tumor. We placed two to three DEs to make one cuboid side. The other side of the cuboid was already on the polar side. (d) A skull base area tumor. We placed two DEs to make one cuboid side. The other side of the cuboid was already on the skull base side. (e) An area involving the hippocampus or amygdala. We directly inserted the DEs into these regions.
Second, we inserted DEs as fence posts according to guidance from the navigation system (Curve™ Neuronavigation System, Brainlab AG, Munich, Germany). The insertion of a DE was performed before dural incision to avoid brain shift. We used two types of DEs made from silicon, one with a 5-mm interval and one with a 10-mm interval. Each DE has six contacts, and the outer diameter of DE was 1.5 mm (Unique Medical Co., Komae, Japan). DEs were chosen depending on the tumor size. If some contacts were located outside the parenchyma, we only used the intraparenchymal contacts for the neurophysiological study.
Long-term DE monitoring
After implantation of electrodes, all patients in Group 2 underwent extra operative monitoring for 2–7 days to capture recurring seizures at least twice. We evaluated which DE and which contact was involved most in the ictal onset zone.
Surgery
For Group 1, we removed the brain tumor using neuronavigation assistance. For Group 2, once we identified the ictal onset zone, we performed both tumor removal and focus resection. After dural incision, resection of the cuboid containing the tumor mass was performed according to fence post guidance.[
Statistical analysis
We used the Mann–Whitney rank-sum test and Fisher’s exact test for statistical analyses of surgical outcomes. Statistical significance was set at P < 0.05. Analysis was done using Sigma Plot 14 (Systat Software, Inc., San Jose, CA, USA).
Ethical approval
Written informed consent was obtained from all patients for procedures performed in this study in accordance with the principles of the Declaration of Helsinki. This study was approved by the Ethics Committee of Seirei Hamamatsu General Hospital.
RESULTS
Clinical information
All clinical information for patients in Groups 1 and 2 are shown in
Fence post DE technique and DE monitoring
We used 50 DEs and 300 contacts for Group 2. No complications resulted from the procedure.
The location of the brain tumors and placement of the DEs for the fence post technique are shown in
In Group 1, seven of 13 patients achieved GTR, five achieved STR, and one achieved PR. In Group 2, 13 of 14 patients achieved GTR and one achieved STR [
The difference between DE contact that suggested epileptogenicity and a contact that did not suggest epileptogenicity was statistically significant (P < 0.001). Shallower contacts showed more epileptogenicity than deeper contacts.
Among the DEs, the location where the DEs were placed did not significantly affect the assessment of epileptogenicity. This procedure did not result in any complications.
Surgical outcome
In Group 1, nine patients were classified as Engel I (70%), two as Engel II (15%), and two as Engel III (15%). In Group 2, all patients were classified as Engel I (100%). The difference between Group 1 and Group 2 in seizure outcome was statistically significant (P = 0.041).
Illustrative case (Patient No. 25)
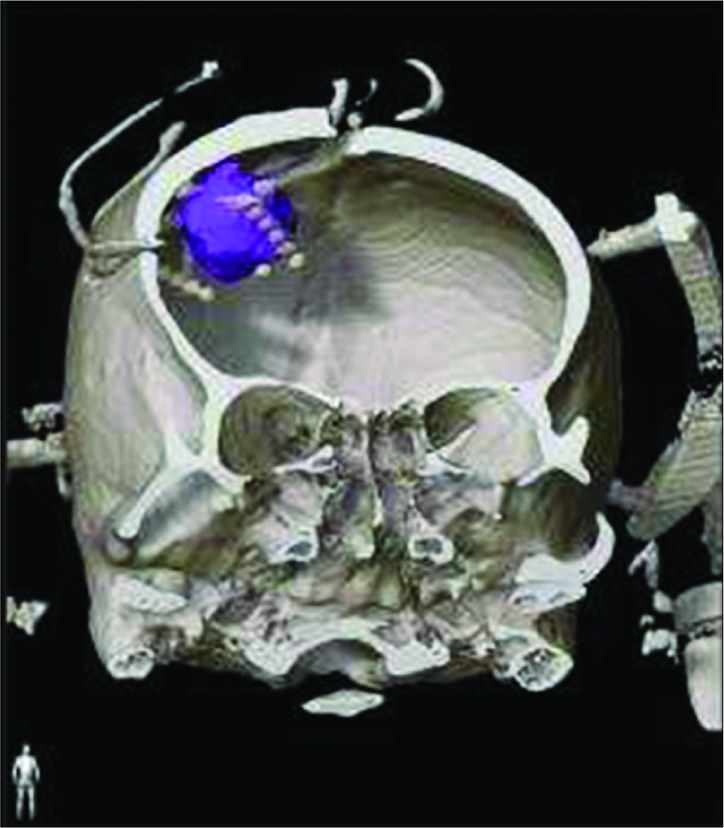
A 57-year-old right-handed man with lung adenocarcinoma had exhibited left face and hand twitching due to metastasis to the right frontal brain. He underwent radiotherapy and chemotherapy for the metastatic brain tumor and was receiving levetiracetam and brivaracetam. Although the metastasis and seizures had remained for 5 months, he exhibited status epilepticus, and MRI at the time revealed tumor regrowth and peritumoral edema. Just before the BTRE operation, he continuously exhibited left arm twitching every 4 h that lasted for an hour.
Due to increased intracranial pressure (IICP), we could not place a subdural grid in this patient. We placed four DEs to form the cuboid containing the metastatic tumor [
Figure 2:
Illustrative case (Patient No. 25). A 57-year-old man with lung adenocarcinoma exhibited left arm and face twitching. The blue area in the right frontal area shows the metastatic tumor. We placed four depth electrodes in the anterior, medial, lateral, and posterior regions of the tumor as fence posts.
DISCUSSION
BTRE requires treatment due to both the brain tumor and epilepsy.[
To control medically refractory epilepsy, invasive monitoring to detect epileptogenicity is required. However, placement of a subdural grid for patients with BTRE is difficult due to IICP.[
The contacts that detected the epileptogenicity were shallower and tended to be located more often in the hippocampus than in other structures. This is probably due to the strong epileptogenicity that is present more in the cortex and hippocampal regions[
CONCLUSION
Using DEs as fence post catheters, we identified the area of epileptic seizure onset and were able to remove the tumor in patients with BTRE, resulting in control of epileptic seizures.
Authors’ contributions
Neurosurgical operation: AF; Acquisition of data: AF, TO, YM, KS, and MN. Analysis and interpretation of data: AF, TO, and HE.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We are thankful to Dr. T. Tanaka for advice regarding surgical technical terms.
References
1. Araki T, Otsubo H, Makino Y, Elliott I, Iida K, Ochi A. Efficacy of dexamathasone on cerebral swelling and seizures during subdural grid EEG recording in children. Epilepsia. 2006. 47: 176-80
2. Battaglia G, Bassanini S, Granata T, Setola V, Giavazzi A, Pagliardini S. The genesis of epileptogenic cerebral heterotopia: Clues from experimental models. Epileptic Disord. 2003. 5: S51-8
3. Birbeck GL, Hays RD, Cui X, Vickrey BG. Seizure reduction and quality of life improvements in people with epilepsy. Epilepsia. 2002. 43: 535-8
4. Du G, Zhou L, Mao Y. Neuronavigator-guided glioma surgery. Chin Med J (Engl). 2003. 116: 1484-7
5. Hildebrand J, Lecaille C, Perennes J, Delattre JY. Epileptic seizures during follow-up of patients treated for primary brain tumors. Neurology. 2005. 65: 212-5
6. Iuchi T, Hasegawa Y, Kawasaki K, Sakaida T. Epilepsy in patients with gliomas: Incidence and control of seizures. J Clin Neurosci. 2015. 22: 87-91
7. Kajiwara K, Yoshikawa K, Ideguchi M, Nomura S, Fujisawa H, Akimura T. Navigation-guided fence-post tube technique for resection of a brain tumor: Technical note. Minim Invasive Neurosurg. 2010. 53: 86-90
8. Kamida T, Fujiki M, Baba H, Ono T, Abe T, Kobayashi H. The relationship between paired pulse magnetic MEP and surgical prognosis in patients with intractable epilepsy. Seizure. 2007. 16: 113-9
9. Karasu A, Kuşcu D, Ofluoğlu AE, Gül G, Kayrak N, Bayindir C. Surgical outcome in hippocampal sclerosis following selective amygdalo-hippocampectomy. Turk Neurosurg. 2008. 18: 374-9
10. Maschio M, Beghi E, Casazza MM, Colicchio G, Costa C, Banfi P. Patterns of care of brain tumor-related epilepsy. A cohort study done in Italian epilepsy center. PLoS One. 2017. 12: e0180470-
11. Metcalfe SE, Grant R. Biopsy versus resection for malignant glioma. Cochrane Database Syst Rev. 2001. 3: CD002034-
12. Mori S, van Zijl PC. Fiber tracking: Principles and strategies a technical review. NMR Biomed. 2002. 15: 468-80
13. Ohue S, Kohno S, Inoue A, Yamashita D, Matsumoto S, Suehiro S. Surgical results of tumor resection using tractography-integrated navigation-guided fence-post catheter techniques and motor-evoked potentials for preservation of motor function in patients with glioblastomas near the pyramidal tracts. Neurosurg Rev. 2015. 38: 293-306
14. Pizzo F, Roehri N, Catenoix H, Medina S, McGonigal A, Giusiano B. Epileptogenic networks in nodular heterotopia: A stereoelectroencephalography study. Epilepsia. 2017. 58: 2112-23
15. Politsky JM. Brain tumor-related epilepsy: A Current review of the etiologic basis and diagnostic and treatment approaches. Curr Neurol Neurosci Rep. 2017. 17: 70-
16. Rudà R, Trevisan E, Soffietti R. Epilepsy and brain tumors. Curr Opin Oncol. 2010. 22: 611-20
17. Spencer SS. Long-term outcome after epilepsy surgery. Epilepsia. 1996. 37: 807-13
18. Tanaka T, Ihara M. Post-stroke epilepsy. Neurochem Int. 2017. 107: 219-28
19. van Breemen MS, Wilms EB, Vecht CJ. Epilepsy in patients with brain tumours: Epidemiology, mechanisms, and management. Lancet Neurol. 2007. 6: 421-30
20. Vertosick FT, Selker RG, Arena VC. Survival of patients with well-differentiated astrocytomas diagnosed in the era of computed tomography. Neurosurgery. 1991. 28: 496-501
21. Vuorinen V, Hinkka S, Färkkilä M, Jääskeläinen J. Debulking or biopsy of malignant glioma in elderly people a randomised study. Acta Neurochir (Wien). 2003. 145: 5-10
22. Yoshikawa K, Kajiwara K, Morioka J, Fujii M, Tanaka N, Fujisawa H. Improvement of functional outcome after radical surgery in glioblastoma patients: The efficacy of a navigation-guided fence-post procedure and neurophysiological monitoring. J Neurooncol. 2006. 78: 91-7