- Department of Neurosurgery, University of Cincinnati, Cincinnati, Ohio, United States,
- Department of Neurosurgery, Azerbaijan Medical University, Baku, Azerbaijan,
- Department of Neurosurgery, University of Baghdad, College of Medicine, Baghdad, Iraq.
Correspondence Address:
Samer S. Hoz, Department of Neurosurgery, University of Cincinnati, Cincinnati, Ohio, United States.
DOI:10.25259/SNI_931_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Samer S. Hoz1, Alkawthar M. Abdulsada2, Mustafa Ismail3, Yara Alfawares1, Jonathan A. Forbes1, Charles J. Prestigiacomo1, Norberto Andaluz1. The functional anatomy of the foramina of Luschka revisited. 04-Nov-2022;13:512
How to cite this URL: Samer S. Hoz1, Alkawthar M. Abdulsada2, Mustafa Ismail3, Yara Alfawares1, Jonathan A. Forbes1, Charles J. Prestigiacomo1, Norberto Andaluz1. The functional anatomy of the foramina of Luschka revisited. 04-Nov-2022;13:512. Available from: https://surgicalneurologyint.com/surgicalint-articles/11973/
Abstract
Background: The German Anatomist Hubert Von Luschka first described the foramina of Luschka (FOL) in 1855 as lateral holes in the fourth ventricle. By his discovery, he refuted previous beliefs about the lateral recess as blind ends of the fourth ventricle, proving the continuity of the ventricular system with the central canal of the spinal cord. In this paper, we question the outline variations of the patent parts of FOL and their consistency, drawing attention to the apparent query of the valvular mechanism of FOL.
Methods: We conducted a literature review in PubMed and Google Scholar databases to review the existing literature describing the history, pertinent anatomy, and function of FOL. In addition, we reviewed the original German book written by Luschka.
Results: While reading the available articles and original works regarding FOL, we noticed the developmental phases through which FOL was discovered, tracking the process from Aristotle till Luschka’s discovery. We also discussed controversies and opinions about FOL’s existence and function.
Conclusion: FOL is halved into two compartments: choroidal and patent. The function of FOL resembles a oneway valve mechanism, and it depends on the patent slit-like part. Luschka had discovered over 20 anatomical structures, including several foramina, confusion in a debate may result from eponyms.
Keywords: Choroid plexus, Foramen of Luschka, Fourth ventricle, Lateral openings, Patency
INTRODUCTION
The foramina of Luschka (FOL) are counted as a considerable microsurgical corridor to the floor of the fourth ventricle. Understanding the patency of FOL can potentially improve tetraventricular microsurgical and neuroendoscopic approaches. It also contributes to the development of transforaminal electrode placement for auditory nerve stimulation and intraoperative neurophysiological monitoring (IONM), especially brainstem auditory evoked potentials.[
A variety of previous revisiting attempts to explore the FOL anatomy, pathology, and surgical significance are reported [
WHO IS LUSCHKA?
The German anatomist Hubert Von Luschka (1820–1875) first studied pharmacology; thence, he was able to join medical college with the financial support of his brothers. However, he well deserved it being eponymous of more than 20 anatomical structures named after him.[
PERTINENT ANATOMY
The superior and inferior limbs of the cerebellopontine fissure form a deltoid space lateral to the pons called the cerebellopontine angle (CPA). CPA is occupied by the CPA’s cistern, where blood vessels and cranial nerves are situated. It is the most frequent site for posterior fossa tumors due to the abundance of arteries which increases the risk of tumor growth in the CPA. Subsequently, the continuous blood supply to the tumors will result in more destructive damage to the surrounding structures.[
Ventricular side of FOL
The fourth ventricle is a cavity with a tent-shaped roof and rhomboid-shaped floor. It is located anterior to the cerebellum, extending from the Sylvian aqueduct to the obex, where it narrows and continues as the central canal of the spinal cord. The union of the floor and roof of the fourth ventricle on either side form the lateral recess, which opens at the CPA by the FOL.[
Choroidal compartment of FOL
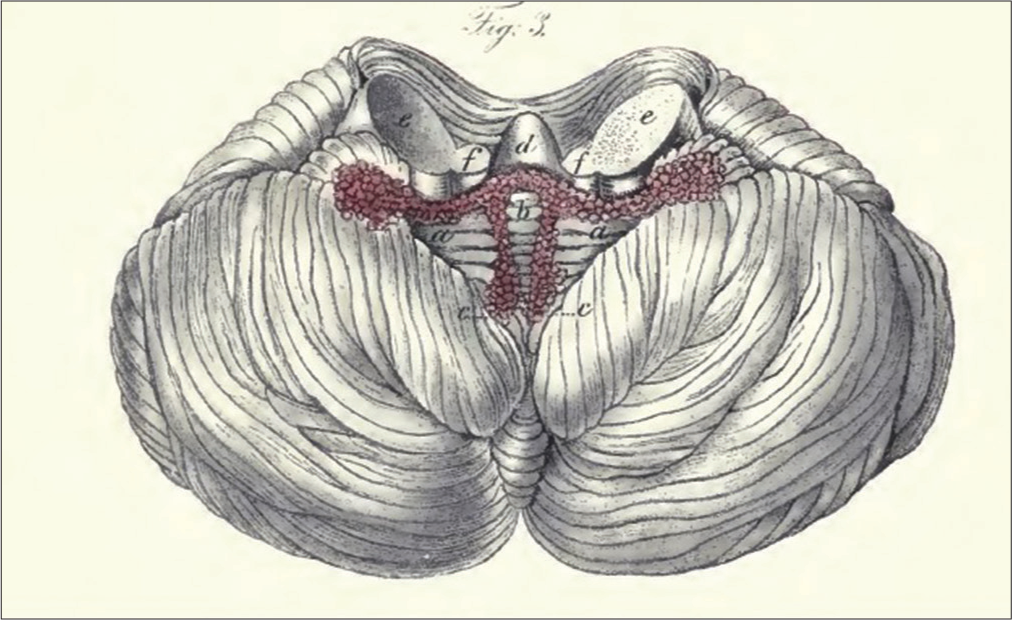
The choroid plexus of the fourth ventricle has T-shaped distribution. Its lateral horizontal section sits laterally in the lateral recess and protrudes outside the FOL, the so-called Bochdalek’s flower basket (BFB) [
Figure 2:
Original illustration by “The Choroid Plexuses of the Human Brain” book, inferior view of the cerebellum after removal of the medulla oblongata and the superior medullary velum to show the T-shape distribution of the BFB ending laterally by the choroidal compartment of FOL. (aa) Horizontal strands of the choroid plexus, (b) connector of the horizontal and longitudinal choroid plexus, (cc) longitudinal strands of the choroid plexus, (d) cerebellar nodule, (ee) middle cerebellar peduncles, and (ff) inferior cerebellar peduncles.
Patent compartment of FOL
The patent part is the crescent-shaped residual medial slit. The patency of FOL depends on the size of BFB and the probability of FOL obstruction, which is inversely proportional to the choroidal part’s size. Membranous obstruction of FOL is caused by remnants of the rhomboid lip and is known as primary obstruction. Primary obstruction usually causes CPA cysts which may develop into diverticular lesions. Congenital syndromes such as Chiari malformation and Walker Dandy can lead to fourth ventricular outlets obstruction resulting in the closure of both the FOL and Magendie simultaneously. Secondary obstruction can be caused by CPA tumors or subarachnoid hemorrhage.[
Cisternal side of FOL
The FOL pours into the cerebellomedullary cistern or cisterna magna, from which ventricular CSF circulation communicates with subarachnoid spaces. Such a connection was not considered until (1747) when Von Haller (1708–1777) described a liquid superficially to the brain and a space between the arachnoid membrane and the pia mater. He assumed that this liquid must have its pathway outward the ventricles but did not describe the mechanism.[
Vessels in the vicinity of FOL
The brainstem receives its vascular supply chiefly from the vertebrobasilar system. The basilar artery gives rise to the superior cerebellar artery most rostrally; it, then, courses laterally and caudally to supply cerebellar hemispheres. A second branch of the basilar artery is the anterior inferior cerebellar artery (AICA).[
The choroid plexus of the fourth ventricle usually receives its blood supply through the AICA, supplying the choroidal part in the cisternal side and the adjacent portion of the lateral recess of the fourth ventricle through the FOL. At the same time, PICA supplies most of the choroidal part in the roof and the foramen of Magendie.[
Cranial nerves in the vicinity of FOL
Cranial nerves from V to XI arise within the margins of the cerebellopontine fissure into the CPA cistern. The CN V exits the brainstem laterally to the pontomedullary sulcus. The entry zone of the vestibulocochlear nerve is 1–2 mm posterior to the trigeminal nerve emergence. The lower part of the CPA contains the CN IX, X, and XI. Perception of cranial nerves in the CPA is of considerable importance in mapping the ambiguous nucleus, facial nucleus, and hypoglossal nucleus during CPA tumors’ surgeries and IONM.[
TRACKING DOWN THE ORIGINAL DESCRIPTION OF FOL
“On either side, the outer angles of the fourth ventricle assume the form of a gutter leading outside, whereby the latter portion of the choroid plexus passes outside the fourth ventricle while the arachnoidea stretches freely over the place in question. The fourth ventricle, therefore by its exterior angles, has open communication with the subarachnoid space.”
That was a part of what Luschka stated in his book The Choroid Plexuses of the Human Brain (Die Adergeflechte des menschlichen Gehirns) (1855) [
DEVELOPMENT
The first description of cerebral cavities was in the 3rd century BC by Aristotle (384-322 BC), as he noticed the central cavity in the brain while studying animals’ cadavers. Then, he came the detailed identification of lateral, third, fourth, and mesencephalic aqueduct through dissections in human cavers by Herophilos of Chalcedon (335-280 BC).[
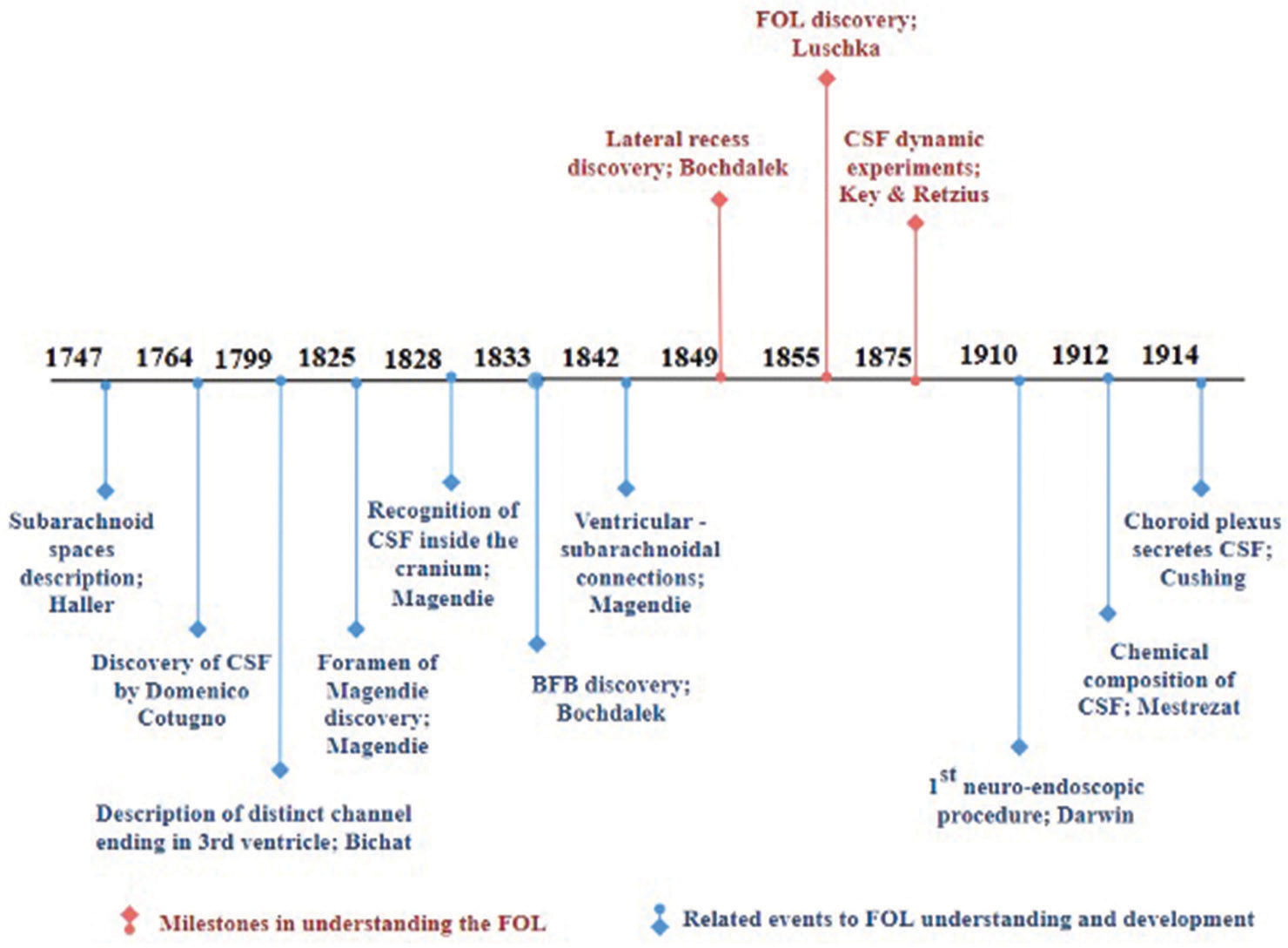
Milestones of FOL anatomical and physiological development
The three main events intimately related to FOL development are represented first by the discovery of the lateral recess, which was described as blind end extensions (1849) by Vincent Alexander Bochdalek (1801–1883). Second, the correction of this description was achieved by the discovery of FOL in (1855) by Hubert Von Luschka (1820–1875). Finally, the ratification of Luschka’s findings was crowned by the dynamic experiments (1875) of Axel Key (1832–1901) and Gustav Magnus Retzius (1842–1919), as they mentioned in their book “Studies in the Anatomy of the Nervous System and the Connective Tissue.”[
Related events to FOL understanding
Ten subordinated events contributed to the milestones of FOL discovery. Albrecht Von Haller (1708–1777) gave the idea of brain liquid and the possibility of a default outlet of this liquid (1747).[
Connections between the ventricles and subarachnoid spaces through the foramen of Magendie were also explained by Magendie (1842). Each discovery has played a key role in neurosurgery hitherto and assisted in improving neurosurgical procedures. Neuroendoscopic procedures emanated, and the first one to fulgurate the choroid plexus was done in 1910 by Vincent Darwin, a urologist. He used the cystoscope to make a third ventriculostomy in two children with hydrocephalus.[
CONTROVERSIES AND OPINIONS REGARDING FOL
Although Monro’s descriptions of the interventricular foramen and ventricular system’s continuity fitted expectations, he denied the continuity between the fourth ventricle and the central canal of the spinal cord. However, the continuous ventricular system was already drawn with wax cast by Leonardo di Vinci at that time.[
The terms “holes” and “foramina” of Luschka are used synonymously in the literature for describing the fourth ventricular lateral apertures. In contrast, the same terms are used to name postglenoid foramen and jugular spurium foramen, discovered by Luschka as well. This may confuse the debate between all the foramina Luschka discovered unless an explanation is mentioned.[
Another contradiction in the literature is the determination of the roof and floor of the lateral recess, a pyramidal extension connecting the fourth ventricle’s cavity with the cerebellomedullary cistern through the FOL.[
FUNCTION OF FOL
The central canal of the neural tube develops into the cerebral ventricular system. During the first trimester of pregnancy, the central canal expands into the ventricles, connected by thinner canals. The fourth ventricle particularly originates from the rhombencephalic portion of the neural tube. FOL appears between the 14th and 26th weeks of gestation without exact known timing. Since the median aperture appears earlier, on the 9–10th week, a bilateral failure in the opening of the lateral recesses should not result in hydrocephalus.[
The anatomy around the FOL is delicate due to the rich vascularity and cranial nerve emergence (CN IX and X) near its opening. The microsurgical anatomy of FOL around the CPA is well described in the literature, but the interior compartment and mechanism of function have poor descriptions.[
The mechanism of the function of the FOL resembles a one-way valve by principle and directionality.[
Magendie carried out human cadaveric experiments to prove CSF bidirectionality by injecting liquids through the subarachnoid space and observing the backflow of the fluid through the median aperture to the fourth ventricle, then to the third ventricle through the Sylvian aqueduct and ending in the lateral ventricles. He also studied the postural outflow drainage by emptying the injection in the lumbar sac.
Luschka conducted seven experiments to prove the continuity of the ventricular system with the subarachnoid spaces and the bidirectionality of the median and lateral foramina. He applied a liquid colored with black ink and injected it at several subarachnoid space levels; by doing so, he proved the CSF continuity, but the directionality of the foramina remained uncertain.[
Key and Retzius used dyed adhesive solutions mixed with paraffin and olive oil. The injected cadavers’ brains were studied only after they were cooled till solidification. They found an uninterrupted stream of CSF in the ventricles, holes, and subarachnoid spaces, but they could not infer the direction as they studied frozen liquid.[
CONCLUSION
FOL is halved into two compartments: choroidal and patent. The function of FOL resembles a one-way valve mechanism dependent on the patent slit-like part. Although the directionality of FOL is believed to be inside-out, it lacks explanation. Hypothesized explanations suggest outer pressure of the choroid plexus.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Barany L, Baksa G, Patonay L, Ganslandt O, Buchfelder M, Kurucz P. Morphometry and microsurgical anatomy of Bochdalek’s flower basket and the related structures of the cerebellopontine angle. Acta Neurochir (Wien). 2017. 159: 1539-45
2. Barany L, Baksa G, Patonay L, Racz G, Ganslandt O, Buchfelder M. Primary obstruction of the foramen of Luschka: Anatomy, histology, and clinical significance. World Neurosurg. 2018. 112: e288-97
3. Boonstra EA, Lorenz K, Porte RJ. The quest for Luschka’s duct: An eponym leading a life of its own?. Dig Surg. 2014. 31: 104-7
4. De MeloMussi AC, da Luz de Oliveira EP, editors. Ventricular anatomy. Comprehensive Overview of Modern Surgical Approaches to Intrinsic Brain Tumors. Ch. 5. Amsterdam: Elsevier Science; 2019. p. 107-18
5. Duque-Parra JE, Barco-Ríos J, García-Aguirre JF. A historical approach to the ventricular system of the brain. Rev Fac Med. 2017. 65: 473-7
6. Engelhardt E. Magendie and Luschka: Holes in the 4th ventricle. Dement Neuropsychol. 2016. 10: 254-8
7. Jean WC, Abdel Aziz KM, Keller JT, Van Loveren HR. Subtonsillar approach to the foramen of Luschka: An anatomic and clinical study. Neurosurgery. 2003. 52: 860-6
8. Johal J, Paulk PB, Oakes PC, Oskouian RJ, Loukas M, Tubbs RS. A comprehensive review of the foramina of Luschka: History, anatomy, embryology, and surgery. Childs Nerv Syst. 2017. 33: 1459-62
9. Karachi C, Le Guérinel C, Brugières P, Melon E, Decq P. Hydrocephalus due to idiopathic stenosis of the foramina of Magendie and Luschka: Report of three cases. J Neurosurg. 2003. 98: 897-902
10. Koval J, Krempaská S. Anatomic logic of the foramen Luschka in neurootologic surgery. Otolaryngol Pol. 2009. 63: 398-402
11. Kuroki A, Møller AR. Microsurgical anatomy around the foramen of Luschka in relation to intraoperative recording of auditory evoked potentials from the cochlear nuclei. J Neurosurg. 1995. 82: 933-9
12. Salma A, Yeremeyeva E, Baidya NB, Sayers MP, Ammirati M. An endoscopic, cadaveric analysis of the roof of the fourth ventricle. J Clin Neurosci. 2013. 20: 710-4
13. Sharifi M, Ciołkowski M, Krajewski P, Ciszek B. The choroid plexus of the fourth ventricle and its arteries. Folia Morphol (Warsz). 2005. 64: 194-8
14. Sharifi M, Ungier E, Ciszek B, Krajewski P. Microsurgical anatomy of the foramen of Luschka in the cerebellopontine angle, and its vascular supply. Surg Radiol Anat. 2009. 31: 431-7
15. Tubbs RS, Vahedi P, Loukas M, Shoja MM, Cohen-Gadol AA. Hubert von Luschka (1820-1875): His life, discoveries, and contributions to our understanding of the nervous system: Historical vignette. J Neurosurg. 2011. 114: 268-72