- Department of Stereotactic and Functional Neurosurgery, Goiânia Neurological Institute, Goiânia, Brazil.
- Department of Neurology, Goiânia Neurological Institute, Goiânia, Brazil.
- Department of Psychiatry, Goiânia Neurological Institute, Goiânia, Brazil.
- Department of Surgery, Division of Neurosurgery, Federal University of Goiás; Goiânia, Brazil.
- Department of Psychiatry, Medical School, Federal University of Goiás; Goiânia, Brazil.
- Department of Neurosciences, Medical School, Pontifical Catholic University of Goiás, Goiânia, Brazil.
Correspondence Address:
Osvaldo Vilela-Filho, Department of Surgery, Division of Neurosurgery, Medical School, Federal University of Goiás, Goiânia, Brazil.
DOI:10.25259/SNI_599_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Osvaldo Vilela-Filho1,4,6, Paulo C. Ragazzo2, Darianne Canêdo6, Uadson S. Barreto6, Paulo M. Oliveira3,5, Lissa C. Goulart4, Manoel D. Reis3, Telma M. Campos3. The impact of subcaudate tractotomy on delusions and hallucinations in psychotic patients. 20-Sep-2021;12:475
How to cite this URL: Osvaldo Vilela-Filho1,4,6, Paulo C. Ragazzo2, Darianne Canêdo6, Uadson S. Barreto6, Paulo M. Oliveira3,5, Lissa C. Goulart4, Manoel D. Reis3, Telma M. Campos3. The impact of subcaudate tractotomy on delusions and hallucinations in psychotic patients. 20-Sep-2021;12:475. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=11117
Abstract
Background: Delusions and hallucinations, hallmarks of the psychotic disorders, usually do not respond to surgical intervention. For many years, the surgical technique of choice for the treatment of refractory aggressiveness in psychotic patients in our Service was amygdalotomy in isolation or associated with anterior cingulotomy. No improvement of hallucinations and delusions was noticed in any of these patients. To improve the control of aggression, subcaudate tractotomy was added to the previous surgical protocol. The main goal of the present study was to investigate the impact of this modified surgical approach on delusions and hallucinations.
Methods: Retrospective analysis of the medical records of psychotic patients presenting with treatment-resistant aggressiveness, delusions, and hallucinations submitted to bilateral subcaudate tractotomy + bilateral anterior cingulotomy + bilateral amygdalotomy in our institution.
Results: Five patients, all males, with ages ranging from 25 to 65 years, followed up by a mean of 45.6 months (17–72 months), fulfilled the inclusion criteria. Delusions and hallucinations were abolished in four of them.
Conclusion: These results suggest that the key element for relieving these symptoms was the subcaudate tractotomy and that the orbitofrontal and ventromedial prefrontal cortices play an important role in the genesis of hallucinatory and delusional symptoms of schizophrenia and other psychoses.
Keywords: Delusions, Hallucinations, Psychosis, Psychosurgery, Schizophrenia, Subcaudate tractotomy
INTRODUCTION
Psychotic disorders, either primary or secondarily determined, are severe and persistent mental disorders causing significant limitations to the social and professional life of the affected individuals.[
Schizophrenia is clinically characterized by positive (psychomotor agitation, aggressiveness, hallucinations, and delusions) and negative symptoms (blunted affect, social isolation, and poverty of speech),[
The therapeutic approach of schizophrenia involves a combination of psychotherapy, psychoactive drugs, and electroconvulsive therapy (ECT).[
When aggressiveness is a significant problem and a source of danger for the own patient and/or for those around him/ her, surgical treatment for the relief of this manifestation may be contemplated.[
When we started operating for aggression in chronic treatment-resistant psychotic patients, we elected bilateral amygdalotomy as the procedure of choice. The rates of failure and recurrence were high, though. In 2001, the use of functional neuroimaging studies to determine the best target to treat varied psychiatric disorders was proposed.[
The goal of this study was to investigate the impact of bilateral limbic leucotomy + bilateral amygdalotomy on treatment-resistant delusions and hallucinations in psychotic patients undergoing surgery for the control of aggressiveness.
MATERIALS AND METHODS
This retrospective, observational, and qualitative study was approved by the Research Ethics Committees of the Pontifical Catholic University of Goiás (technical report # 239.724) and of the Clinics Hospital of the Federal University of Goiás (technical report # 599.636-0). The primary source of data was the medical records of patients with chronic psychotic disorders operated for aggressiveness at the Goiânia Neurological Institute between September 2003 and December 2011.
The diagnosis was established pursuant to the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition.[
Refractoriness was defined as the presence of frequent psychomotor agitation events associated to self- and/or hetero-aggressiveness, with danger to him/herself and/or to others, despite ongoing optimized treatment with at least three neuroleptic drugs (haloperidol, clozapine, risperidone, ziprasidone, olanzapine, zuclopenthixol, and aripiprazole), in association with benzodiazepines (lorazepam, clonazepam, clobazam, and cloxazolam), nonbenzodiazepine inhibitors of anxiety and impulsivity (propranolol, buspirone, and citalopram), and mood stabilizers (sodium valproate, divalproex sodium, lithium carbonate, topiramate, and oxcarbazepine), all of these drugs in optimal dosages and for a period considered to be adequate, as certified by two independent psychiatrists and one behavioral neurologist. Besides, the disease must have lasted a minimum of 5 years and be the cause of a significant impact on the patient’s global functioning and quality of life. Although offered to all patients, ECT was not considered a required criterion for determining refractoriness.
Each patient referred from psychiatric clinics not pertaining to the Goiânia Neurological Institute was submitted to reevaluation by two psychiatrists and one behavioral neurologist to confirm surgical indication. The functional neurosurgeon did not take any part in patient selection for surgery.
An informed consent form signed by the patient or by their guardians was obtained in all cases after meticulous explanation of the technical aspects, risks, and benefits of the surgical procedure. Patient privacy was protected with routine measures to prevent patient and family identification.
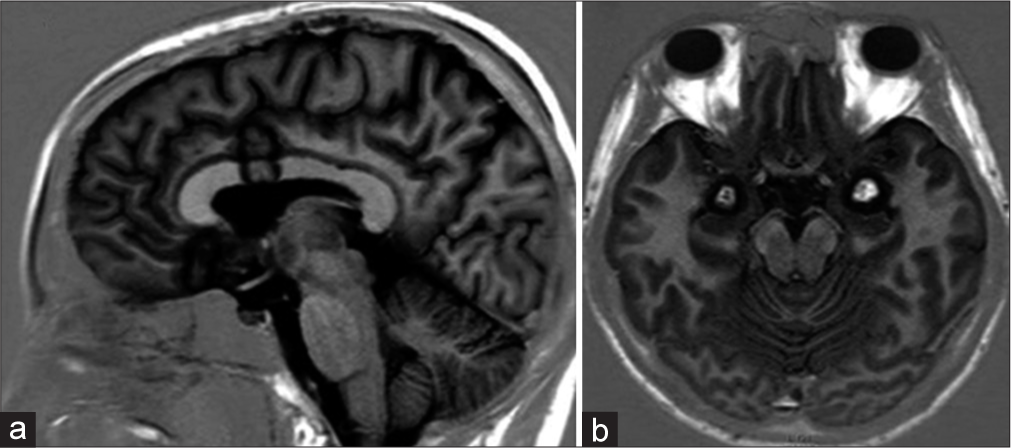
During the preoperative period, a neuropsychological evaluation was performed in every case and all the patients underwent magnetic resonance (MR) and single-photon emission computed tomography (SPECT) of the brain. MR or computed tomography (CT) was repeated postoperatively within the first 48 h to determine the adequacy of the position and size of the surgical lesions, and to exclude surgical complications.
The clinical follow-up was done in a systematic manner by at least one of the psychiatrists, the behavioral neurologist, and the functional neurosurgeon. Follow-up visits were scheduled every 3 months during the 1st year, every 6 months during the 2nd year, and, after that, every year or as needed. In the meantime, the patient was also accompanied by his/her referring physician, if he/she was not a member of our institution’s staff.
Postoperatively, as part of our protocol, medications remained unchanged for a minimum of 6 months so as to better evaluate the impact of surgery on the clinical features.
To evaluate the changes in quality of life, aggressiveness, psychomotor agitation, delusions, and hallucinations, the authors relied on spontaneous reports from the patients and/or from their close relatives and on their answers to the questions posed by the psychiatrist or neurologist regarding these issues in each follow-up visit.
Surgical technique
The surgical procedure was performed under general anesthesia. Short-term neuromuscular blockers were used only during the induction of anesthesia so as to not interfere with the assessment of motor responses during electrical stimulation. After the placement of the stereotactic frame, CT axial slices were obtained and merged with the inversion recovery axial MR slices obtained on the previous day. Targets’ coordinates were determined on these fused images, as follows:
Amygdala
The index target is taken at the most central part of the amygdala, as observed on reconstructed coronal slices. Additional targets are established 3.0 mm superior, inferior, anterior, posterior, medial, and lateral to the index target (five trajectories and seven targets).
Anterior cingulate gyrus
Using reconstructed sagittal and coronal slices, the index target is determined 20.0 mm posterior to the anterior wall of the lateral ventricle (y), 6.0 mm from the interhemispheric fissure (x), and at the interface between the corpus callosum and the anterior cingulate gyrus (z). Two additional targets are established 4.0 mm superior and inferior to the index target. A second mirror trajectory is performed 3.0 mm anterior to the first one (two trajectories and six targets).
Subcaudate area
Using axial and reconstructed sagittal slices, the index target is established at mid-distance between the corpus callosum and the floor of the anterior fossa (z), 7.0 mm from the interhemispheric fissure (x), and 15.0 mm in front of the anterior wall of the third ventricle (y). Four additional targets, 3.0 and 6.0 mm superior and inferior to the index target, are used. A second mirror trajectory, 5.0 mm lateral to the first one (12.0 mm from the interhemispheric fissure), is performed (two trajectories and 10 targets).
Using a 1.27 mm in diameter and 4.0 mm bare tip electrode, electrical stimulation (3.0 V/100 Hz) is performed at all targets. In the absence of adverse motor responses, radiofrequency thermocoagulation is performed with 85°C/60 s.
Inclusion criteria
Psychotic patients with a disease duration over 5 years, presenting with treatment-resistant aggressiveness, associated to refractory delusions and hallucinations, who were treated with bilateral limbic leucotomy + bilateral amygdalotomy, and not submitted to additional further surgery aiming to achieve a broader disconnection of the prefrontal cortex, such as anterior capsulotomy.
Exclusion criterion
Previous surgery for the treatment of aggressiveness.
RESULTS
On reviewing the medical records, the investigators found five patients with chronic psychotic disorder that fulfilled the inclusion criteria. All patients were male with ages ranging from 25 to 65 years (35.4 ± 17.3 years), two of them already treated with several ECT sessions. These patients were followed up by a mean of 45.6 ± 21.38 months (17– 72 months) and are summarized in [
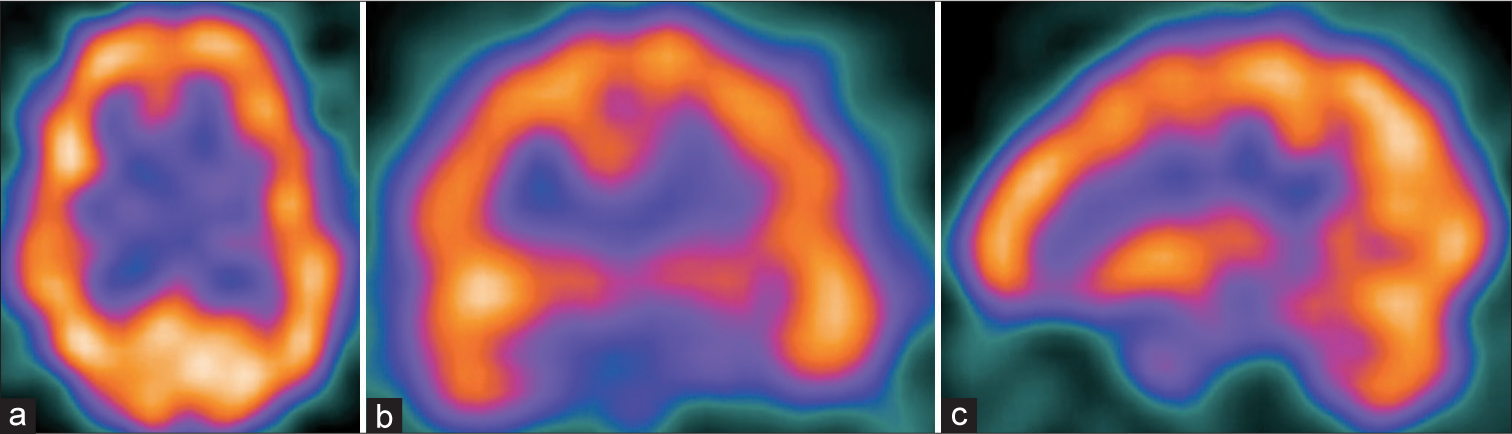
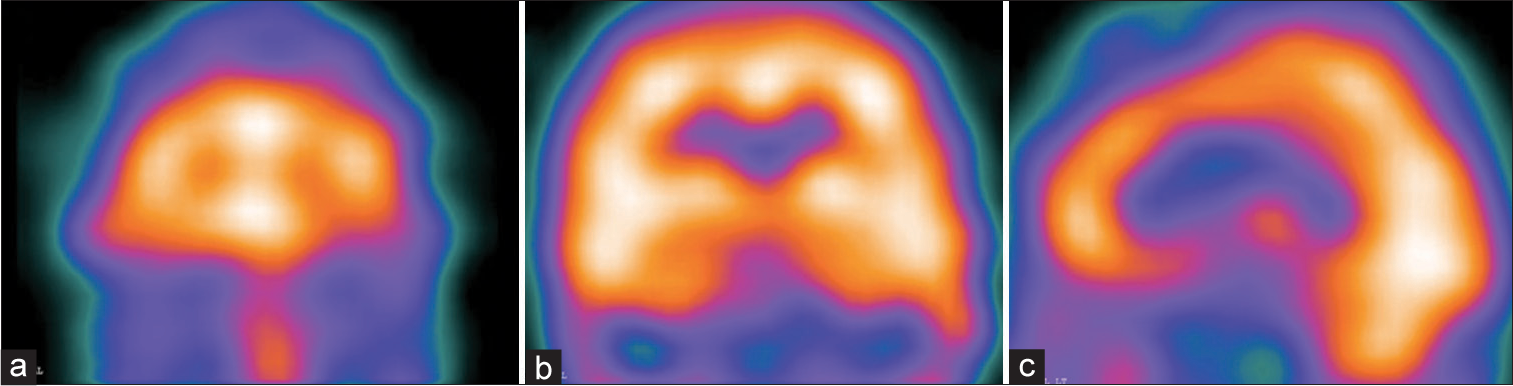
Preoperatively, all the patients underwent SPECT [
According to reports given by the patients and their close relatives, four of them presented a significant improvement in the quality of life, in the social relationships, and complete resolution of the delusions, hallucinations, and aggressiveness. The delusional/hallucinatory symptoms initially declined during the first 3 postoperative days and then completely disappeared afterwards. Only a mild improvement was observed in the remaining patient, though.
Recurrence of aggressiveness occurred after 1 year in one of the patients (patient 2), although he remained free from delusions and hallucinations [
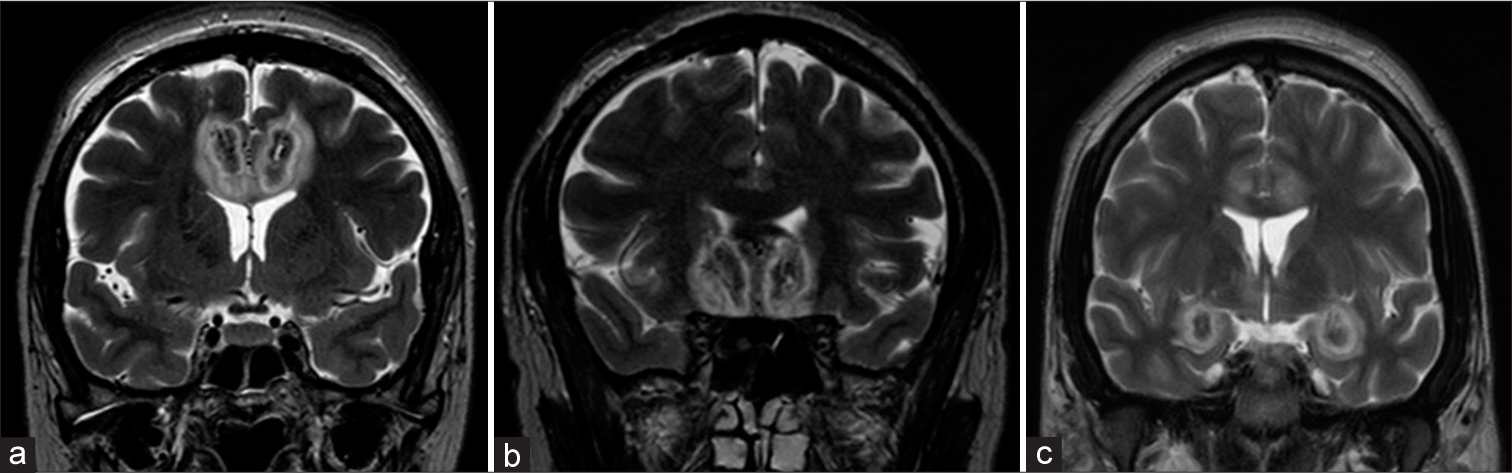
Postoperative MR (four patients) or CT (one patient) was performed within the first 48 h in all patients. The images showed that the location and size of the surgical radiofrequency lesions were adequate in every case [
Surgical complications are listed in [
DISCUSSION
The authors elected schizophrenia as the paradigm for the study of delusional and hallucinatory symptoms, also present in other psychoses.
According to the most accepted hypothesis, the hyperactivity of the mesocorticolimbic dopaminergic system, which extensively modulates the ventromedial and orbitofrontal prefrontal regions, is the determining factor for the appearance of psychosis and other symptoms of schizophrenia.[
Another line of evidence derives from the studies of connectivity of large-scale brain networks.[
Summing up the aforementioned findings, in schizophrenia, the mesocorticolimbic dopaminergic system, which is hyperactive, would over activate the ventromedial and orbitofrontal prefrontal cortices; these areas, in turn, would over excite the anterior portions of the default-mode network, with whom they are functionally hyperconnected, determining the appearance of delusions and hallucinations. Corroborating these observations, the preoperative SPECT, available for analysis in four of our patients, showed hyperperfusion in the prefrontal area in all [
Previously, in our Service, we had performed bilateral amygdalotomy in isolation or associated to bilateral anterior cingulotomy in 47 aggressive patients, 21 diagnosed as schizophrenics; however, these surgical strategies did not provide alleviation of the delusions and hallucinations in any of those presenting these symptoms (unpublished data).
Taken as a whole, these results suggest that the key element for relieving delusions and hallucinations was the bilateral subcaudate tractotomy, which was associated to bilateral anterior cingulotomy and bilateral amygdalotomy in the five patients reported in the present study. We hypothesize that the subcaudate tractotomy acts by interrupting the efferences from the orbitofrontal and ventromedial prefrontal cortices to the anterior portions of the default-mode network, as well as the dopaminergic input from the nucleus accumbens to those areas.
Obviously, it is impossible to completely disregard the contribution of amygdalotomy and anterior cingulotomy in these results, even more when one considers the prominent role played by the amygdala and the anterior cingulate gyrus in the mesocorticolimbic circuit. In fact, both procedures may have potentialized the effect of subcaudate tractotomy, although, alone, uncapable to alleviate delusions and hallucinations.
One of our patients (patient 5,
After reviewing the literature regarding the surgical treatment of psychiatric disorders, it was observed that patients with schizophrenia were included in a number of studies.
Cox and Brown reported the results of bilateral limbic leucotomy + amygdalotomy for the treatment of schizophrenia and aggressive states. A significant improvement was observed in the schizophrenic symptoms of 32 patients.[
Ballantine reported that delusions and hallucinations may be modified with stereotactic lesions but are seldom abolished.[
In conclusion, reports of relief (never complete) of delusions and hallucinations in psychotic patients who underwent surgery are sporadic and inconsistent. Moreover, we have not been able to identify any new study on this topic in the English published literature from 1988 to 2013 (pubmed.com).
It is worthwhile highlighting that the surgical techniques used in the aforementioned studies are somewhat different from the techniques currently used. The anterior cingulotomy and amygdalotomy were based on the ventriculography, and the subcaudate tractotomy, essentially, on X-ray images of the skull. In addition, the technique used to create the subcaudate tractotomy lesion normally required implantation of yttrium 90 seeds; the lesion produced was approximately 20 mm long, 10 mm wide, and 5.0 mm high and its medial limit was 7.0–10 mm from the interhemispheric fissure.[
More recently, Liu et al., in a well-structured study, reported their results in 100 schizophrenic patients submitted to bilateral anterior capsulotomy followed up for 2 years.[
Our results, along with those of Liu et al., suggest that the orbitofrontal and ventromedial prefrontal cortices play an important role in the genesis of the hallucinatory and delusional symptoms of schizophrenia and other psychoses. To confirm this hypothesis, new and more in-depth structural (tractography) and functional (fMR and PET) neuroimaging studies are necessary. Once confirming this hypothesis, chronic electrical stimulation of the orbitofrontal and/ or the ventromedial prefrontal cortex emerges as tentative procedures for the surgical control of these manifestations. In the era of neuromodulation, the benefits thus gained could be substantial, in view of the high prevalence of psychoses and the fact that they are treatment resistant in a significant percentage of patients.
This study presents considerable limitations. Our sample is small, no control group was included, and it was not possible to perform a double-blind study in view of the inherent characteristics of the procedure and of the nature of this study (retrospective). Besides, no formal clinical scales were used to evaluate quality of life, delusions, hallucinations, and aggressiveness.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to express their gratitude to Paulo Verlaine B. Azevêdo, MD, PhD, for his valuable suggestions.
References
1. 2. Ballantine HT, Wilkins RH, Rengachary SS.editors. Neurosurgery for behavioral disorders. Neurosurgery. New York: McGraw-Hill; 1985. p. 2527-37 3. Ballantine HT, Bouckoms AJ, Thomas EK, Giriunas IE. Treatment of psychiatric illness by stereotactic cingulotomy. Biol Psychiatry. 1987. 22: 807-19 4. Busatto Filho G. Structural and functional anatomy of schizophrenia: Neuropathological and neuroimaging findings. Rev Bras Psiquiatr. 2000. 22: 9-11 5. Camchong J, MacDonald AW, Bell C, Mueller BA, Lim KO. Altered functional and anatomical connectivity in schizophrenia. Schizophr Bull. 2011. 37: 640-50 6. Charlson FJ, Ferrari AJ, Santomauro DF, Diminic S, Stockings E, Scott JG. Global epidemiology and burden of schizophrenia: Findings from the global burden of disease study 2016. Schizophr Bull. 2018. 44: 1195-203 7. Cox AW, Brown MH, Sweet WH, Obrador S, Martin-Rodriguez JG.editors. Results of multi-target limbic surgery in the treatment of schizophrenia and aggressive states. Neurosurgical Treatment in Psychiatry, Pain and Epilepsy. Baltimore: University Park Press; 1977. p. 469-82 8. Creese I, Burt DR, Snyder SH. Dopamine receptor binding predicts clinical and pharmacological potencies of anti-schizophrenic drugs. Science. 1976. 192: 481-3 9. Da Costa DA. The role of psychosurgery in the treatment of selected cases of refractory schizophrenia: A reappraisal. Schizophr Res. 1997. 28: 223-30 10. Dickerson FB. Cognitive behavioral psychotherapy for schizophrenia: A review of recent empirical studies. J Nerv Ment Dis. 2000. 188: 187-201 11. Goktepe EO, Young LB, Bridges PK. A further review of the results of stereotactic subcaudate tractotomy. Br J Psychiatry. 1975. 126: 270-80 12. Hertz MI, Liberman RP, Lieberman JA. Practice guideline for the treatment of patients with schizophrenia. Am J Psychiatry. 1997. 154: 1-63 13. Hussain ES, Freeman H, Jones RA. A cohort study of psychosurgery cases from a defined population. J Neurol Neurosurg Psychiatry. 1988. 51: 345-52 14. Jiang CC. A preliminary report on stereotactic multi-target limbic leucotomy. Zhonghua Shen Jing Jing Shen Ke Za Zhi. 1989. 22: 152-4 15. Karbasforoushan H, Woodward ND. Resting-state networks in schizophrenia. Curr Top Med Chem. 2012. 12: 2404-14 16. Kegeles LS, Abi-Dargham A, Frankle WG, Gil R, Cooper TB, Slifstein M. Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch Gen Psychiatr. 2010. 67: 231-9 17. Kelly D, Richardson A, Mitchell-Heggs N, Laitinen LV, Livingston KE.editors. Technique and assessment of limbic leucotomy. Surgical Approaches in Psychiatry. Baltimore: University Park Press; 1973. p. 165-73 18. Knight G, Hitchcock E, Laitnen L, Vaernet K.editors. Bifrontal stereotaxic tractotomy in the substantia innominata: An experience of 450 cases. Psychosurgery. Springfield: Charles C. Thomas; 1972. p. 269-71 19. Liu W, Hao Q, Zhan S, Li D, Pan S, Li Y. Long-term follow-up of MRI-guided bilateral anterior capsulotomy in patients with refractory schizophrenia. Stereotact Funct Neurosurg. 2014. 92: 145-52 20. Meltzer HY, Stahl SM. The dopamine hypothesis of schizophrenia: A review. Schizophr Bull. 1976. 2: 19-76 21. Menon V. Large-scale brain networks and psychopathology: A unifying triple network model-review. Trends Cogn Sci. 2011. 15: 483-506 22. Mitchell-Heggs N, Kelly D, Richardson A. Stereotactic limbic leucotomy: A follow-up at 16 months. Br J Psychiatry. 1976. 128: 226-40 23. Pádua AC, Gama CS, Lobato MI, Abreu PB, Cordioli AV.editors. Schizophrenia: Guidelines and algorithm for pharmacological treatment. Psicofármacos: Consulta Rápida. Porto Alegre: Artmed; 2005. p. 343-51 24. Price G, Cercignani M, Parker GJ, Altmann DR, Barnes TR, Barker GJ. Abnormal brain connectivity in first-episode psychosis: A diffusion MRI tractography study of the corpus callosum. Neuroimage. 2007. 35: 458-66 25. Pull C, Maj M, Sartorius N.editors. Diagnosis of schizophrenia: A review. Esquizofrenia. Porto Alegre: Artmed; 2005. p. 13-70 26. Roeder F, Muller D, Orthner H.editors. Stereotactic treatment of psychoses and neuroses. Berlin Symposium on Special Topics in Stereoencephalotomy. Stuttgart: Hippokrates; 1971. p. 82-102 27. Rotarska-Jagiela A, van de Ven V, Oertel-Knochel V, Uhlhaas PJ, Vogeley K, Linden DE. Resting-state functional network correlates of psychotic symptoms in schizophrenia. Schizophr Res. 2010. 117: 21-30 28. Seeman P. All roads to schizophrenia lead to dopamine supersensitivity and elevated dopamine D2 high receptors. CNS Neurosci Ther. 2011. 17: 118-32 29. Shergill SS, Brammer MJ, Williams SC, Murray RM, McGuire PK. Mapping auditory hallucinations in schizophrenia using functional magnetic resonance imaging. Arch Gen Psychiatry. 2000. 57: 1033-8 30. Shirakawa I. General aspects of the management of schizophrenic patients. Rev Bras Psiquiatr. 2000. 22: 56-8 31. Silva RC. Schizophrenia: A review. Psicol USP. 2006. 17: 263-85 32. Simons JS, Garrison JR, Johnson MK. Brain mechanisms of reality monitoring-review. Trends Cogn Sci. 2017. 1669: 1-12 33. Srmaka M, Pogády P, Csoková Z, Pogády J. Long-term results in patients with stereotaxic surgery for psychopathologic disorders. Bratisl Lek Listy. 1992. 93: 364-6 34. Ström-Olsen R, Carlisle S. Bi-frontal stereotactic tractotomy: A follow-up study of its effects on 210 patients. Br J Psychiatry. 1971. 118: 141-54 35. Tooth JC, Newton MP.editors. Leucotomy in England and Wales 1942-1954. Reports on Public Health and Medical Subjects No 104. London, UK: Her Majesty’s Stationery Office; 1961. p. 36. van Zelst C. Stigmatization as an environmental risk in schizophrenia: A user perspective. Schizophr Bull. 2009. 35: 293-6 37. Vikki J, Sweet WH, Obrador S, Martin-Rodriguez JG.editors. Late psychological and clinical effects of subrostral cingulotomy and anterior mesoloviotomy in psychiatric illness. Neurosurgical Treatment in Psychiatry, Pain and Epilepsy. Baltimore: University Park Press; 1977. p. 253-9 38. Vilela Filho O, Carneiro Filho O, Souza HAO, Machado DC, Rodrigues Filho S, Campos JA. SPECT based tailoring of psychosurgical procedures: Is it possible?. Stereotact Funct Neurosurg. 2001. 76: 256-61 39. Vilela-Filho O. Commentary: Amygdala and hypothalamus: Historical overview with a focus on aggression. Neurosurgery. 2019. 85: E1-3 40. Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci USA. 2009. 106: 1279-84






Dr. Miguel A. Faria
Posted September 21, 2021, 8:02 am
This is an important paper on neuropsychiatry. It is of interest the authors found no improvement in patients with aggressiveness, hallucinations, and delusions treated with amygdalotomy with or without anterior cingulotomy. This is inconsistent with the work of Drs. Vernon H. Mark and Frank R. Ervin in their classic but controversial book Violence and the Brain (1970). For them, amydalotomy was effective in reducing aggressiveness. So, It was time that this issue be reviewed, treatments clarified, and hopefully expansion in the horizons of knowledge.
Out of 5 psychotic patients withe delusions, hallucinations, and treatment-resistant aggressiveness, 4 patients were successfully treated with abolition of their delusions, hallucinations, and aggressiveness. The procedures “eventually became: bilateral limbic leucotomy (i.e., anterior cingulotomy and subcaudate tractomy) and bilateral amygdalotomy on treatment-resistant delusions and hallucinations in psychotic patients undergoing surgery for the control of aggressiveness.”
After providing two good tables and writing a good discussion, the authors conclude that bilateral subcaudate tractotomy was the essential surgical treatment for relieving these symptoms and that the orbitofrontal and ventromedial prefrontal cortices play important roles in the genesis of hallucinations and delusions in psychotic patients, including patients with schizophrenia. The authors should be congratulated not only on their results but also on the presentation of their elegant study.