- Departments of Neurosurgery, Helsinki University Hospital, University of Helsinki, Helsinki, Finland,
- Departments of Neurosurgery, Umberto I General Hospital, Università Politecnica delle Marche, Ancona,
- Departments of Neurosurgery, Ospedali Riuniti Marche Nord, Pesaro, Italy,
- Department of Pathology, University of Helsinki and HUSLAB, Helsinki University Hospital, Helsinki, Finland,
- Juha Hernesniemi International Center for Neurosurgery, Henan Provincial People’s Hospital, Zhengzhou, China.
Correspondence Address:
Joham Choque-Velasquez
Departments of Neurosurgery, Helsinki University Hospital, University of Helsinki, Helsinki, Finland,
Juha Hernesniemi International Center for Neurosurgery, Henan Provincial People’s Hospital, Zhengzhou, China.
DOI:10.25259/SNI-180-2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Joham Choque-Velasquez, Julio C. Resendiz-Nieves, Behnam Rezai Jahromi, Roberto Colasanti, Rahul Raj, Kenneth Lopez-Gutierrez, Olli Tynninen, Mika Niemelä, Juha Hernesniemi. The microsurgical management of benign pineal cysts: Helsinki experience in 60 cases. 19-Jun-2019;10:103
How to cite this URL: Joham Choque-Velasquez, Julio C. Resendiz-Nieves, Behnam Rezai Jahromi, Roberto Colasanti, Rahul Raj, Kenneth Lopez-Gutierrez, Olli Tynninen, Mika Niemelä, Juha Hernesniemi. The microsurgical management of benign pineal cysts: Helsinki experience in 60 cases. 19-Jun-2019;10:103. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=9381
Abstract
Background: Microsurgical resection represents a well-accepted management option for symptomatic benign pineal cysts. Symptoms such as a headache, hydrocephalus, and visual deficiency are typically associated with pineal cysts. However, more recent studies reported over the past years have characterized additional symptoms as a part of the clinical manifestation of this disease and represent additional indications for intervention.
Methods: We present a retrospective review of patients with histologically confirmed benign pineal cysts that were operated on in our department between 1997 and 2015. A demographic analysis, evaluation of preoperative status, surgical treatment, as well as immediate and long-term clinical and radiological outcomes were conducted.
Results: A total of 60 patients with benign pineal cysts underwent surgery between 1997 and 2015. Gross total resection was achieved in 58 cases. All patients except one improved in their clinical status or had made a full recovery at the time of the last follow-up. The key steps for surgical resection of pineal cysts are reported, based on an analysis of representative surgical videos.
Conclusions: We describe in this paper one of the largest series of microsurgically treated pineal cysts. In our opinion, judicious microsurgery remains the most suitable technique to effectively deal with this disease.
Keywords: Microneurosurgery, Pineal cysts, Pineal region lesions, Sitting position, Supracerebellar infratentorial approach
INTRODUCTION
Benign pineal cysts are usually incidental findings on magnetic resonance imaging (MRI). Microsurgical resection represents a well-accepted option for symptomatic benign pineal cysts.[
The supracerebellar infratentorial paramedian approach in the “sitting praying position,” a well previously described variant of the classic sitting position,[
In this paper, we report the surgical outcome of our series of benign pineal cysts encountered in Helsinki, which constitutes one of the largest cohorts investigated, while also describing in detail “tips and tricks” which allow for simple, safe, and effective microsurgical management of these lesions. Moreover, we analyze in detail the surgical indications for pineal cyst surgery.
METHODS
Patient population
We retrospectively reviewed the patients with histologically confirmed benign pineal cysts that were operated on in our department between 1997 and 2015. A demographic analysis, evaluation of preoperative status, and surgical treatment were conducted together with an assessment of immediate and long-term clinical and radiographic outcomes. Finally, the currently available patient information was reviewed in the Finnish Population Registry (as of July 2018) to determine the current clinical status of individuals. Chi-Square test and ANOVA test were used for the statistical analysis of qualitative and quantitative variables.
Pineal cyst surgery
The protocol for surgical treatment of pineal cysts in Helsinki was defined by the neuro-oncological and neurosurgical teams on the basis of the existing literature in 1997, when the senior author (JH) became the chairman of the department. The protocol was subsequently revised according to most recent data.
The following criteria included were used as an indication for surgery: (1) vertical gaze paralysis or double vision of unknown origin with midbrain compression observed in the imaging; (2) obstructive hydrocephalus; (3) cyst growth on sequential imaging studies with 1.5–2 cm as a cut of size to intervene; (4) presence of a solid component suggestive of a pineal tumor; and (5) largest cyst diameter >20 mm, associated to unspecific symptoms such as headache, visual disturbances, psychiatric symptoms, or sensory deficits among others. The threshold size of 20 mm to surgically treat pineal cysts in this last group was determined based on the average size of symptomatic pineal cysts published in literature.[
The goal of surgery was always to achieve a total removal of the pineal cyst. In fact, our and other surgical teams experiences demonstrated that partial resection might still progress to obstruction of the cerebrospinal fluid (CSF) pathway, and it has been reported that puncture of the cyst alone results in less effective treatment.[
Positioning and approach
The “sitting praying position” represents our standard position.[
Risks and benefits as well as the exclusion criteria for the sitting position have been reported on detail previously.[
Regarding meticulous surgical access, the reported supracerebellar infratentorial approach is our standard approach.[
Microneurosurgery
In addition, we carried out a retrospective review of microsurgical videos of the past 22 consecutive cases of benign pineal cysts operated by the senior author (JH), with the aim to identify the key microsurgical steps and potential pitfalls of the procedure. In general, neuromonitoring was not employed for these cases and endoscopic-assisted procedures were not pursued either. Gross total resection of the lesions was achievable in most cases.[
Ethical aspects and analysis of the data
Following Institutional Ethics Board approval (#HUS/2772/2017), we accessed the hospital register and an authorized coauthor (R.R.) accessed the Finnish Population Register. We collected information about the clinical status, histopathology, imaging, and surgical treatment and follow-up from the medical records. IMPAX version 6.5.5.1608 (Agfa, Mortsel, Belgium) was employed for qualitative radiological analysis. Cranial computed tomography (CT) in patients with head trauma or drowsiness was useful to reveal incidental pineal cysts or acute hydrocephalus, respectively. Preoperative MRI studies were used to describe anatomic features of the cyst such as the size, the presence of solid components, contrast enhancement, midbrain compression, and presence of hydrocephalus. T1-weighted images (slice thickness 3–7 mm), T2-weighted images 5–7 thick, fluid-attenuated inversion recovery (FLAIR), and post contrast T1 MRI sequences had been obtained with spin-echo sequences through a number of 0.2 T, 1T, 1.5T, and 3T MRI machines. Preoperative aqueductal CSF flow measurements by phase contrast MR were not acquired. The cyst size was determined in sagittal anteroposterior dimension (SAPd), in sagittal craniocaudal dimension (SCCd), and in axial width (AW). For the postoperative radiological outcome, we reviewed immediate postoperative cranial CT scans without contrast obtained within the first 24 h, and long-term MRI studies acquired after few weeks from surgery. When postoperative MRI showed complete pineal cyst removal, further MRI studies were performed only if the patient experienced new symptoms.
RESULTS
The characteristics of 60 patients (44 females and 16 males) with a mean age of 29.1 ± 12.4 (4–55) years, as our cohort of surgically treated pineal cysts, are presented in
Most frequently reported preoperative symptom was a headache which showed a variety of characteristics (“exercise headache” after physical activity in some patients, long-term migraine with acute exacerbations for several years before surgery, or intense headaches due to acute or subacute hydrocephalus events in a third group). Visual disturbances were reported with “nonspecific” symptoms, reduction of the visual acuity, and tubular vision. Unfortunately, data from formal neuro-ophthalmology examinations were only partially available for the current project. As mentioned above, only double vision was considered a symptom related to the compressive effect of the tectum. Details about symptoms in our study group are described in
Selection criteria for surgery
Indications for surgery are listed in [
Criterion 1 was compressive effect on the tectal plate presenting as double vision. This was the surgical indication in seven patients. However, only a patient, with a pineal cyst and a few months history of double vision, quickly worsened with a sudden temporary visual deficit associated with headache and was operated due to this specific reason. The other six patients showed more than one criterion that was considered an indication for surgery.
Criterion 2 was obstructive hydrocephalus. This was found in 21 of our patients, and nine of them were operated on for this criterion alone. Only a patient had a ventriculoperitoneal shunt placed before surgical excision of the cyst. This patient, that still had persistent headache and nausea after a ventriculoatrial shunt without evidence of ventriculomegaly on multiple postoperative CT scan evaluation, required cyst removal for complete recovery and resolution of the symptoms. None but one of the other patients developed hydrocephalus during follow-up or required a shunt after surgical excision of the pineal cyst. One patient with hydrocephalus and a small postsurgical residual cyst subsequently underwent a third ventricle endoscopic fenestration without complications. Finally, one patient who had hydrocephalus preoperatively and who underwent a stereotactic procedure for aspiration of the cyst content did not require any future procedure. Rapidly progressive hydrocephalus events were associated with severe headache, nausea, vomit, double vision, papilledema, and unconsciousness in few days, while patients with less progressive hydrocephalus presented headache, double vision, ataxia, vertigo, incontinence, hand cramps, panic attacks, psychotic symptoms, sensory deficits, and neurocognitive and memory deficiencies. Only a previously healthy patient was reported to have sudden nausea, vomiting, headache, and visual disturbances in the lateral gaze associated with acute hydrocephalus due to a 1.8 cm pineal cyst.
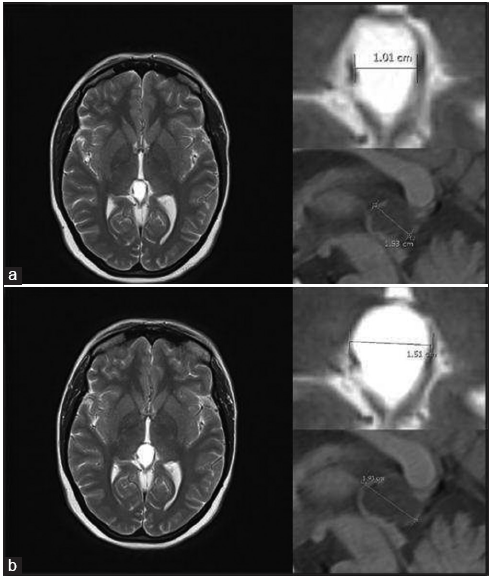
It is worth noting that six patients presented with symptoms associated with physical activity such as moderate to intense exertional headache, vertigo, and nausea. They were considered as cases with probable fluctuant hydrocephalus. The average size of these six cysts was 1.75 ± 0.18 (1.5–2) cm in SAPd, 1.05 ± 0.20 (0.7–1.3) cm in SCCd, and 1.36 ± 0.34 (0.8–1.8) cm in AW. In two cases, data were not available, and in the remaining 13 pineal cysts associated with hydrocephalus, the average size was 2.49 ± 0.96 (1.3–5) cm in the SAPd, 1.62 ± 0.85 (0.9–3.9) cm in SCCd, and 1.92 ± 0.48 (1.4–2.9) cm in AW.
Criterion 3 was cyst enlargement during follow-up and was observed in 10 patients (9 females) with ages ranging between 15 and 37 years old. The average size of the cysts at the last preoperative evaluation was 1.91 ± 0.30 (1.4–2.4) cm in SAPd, 1.27 ± 0.38 (0.6–1.9) cm in SCCd, and 1.46 ± 0.45 (0.8–2) cm in AW. The average size of the cysts at the initial preoperative evaluation was 1.58 ± 0.26 (1.2–2.0) cm in SAPd, 1.06 ± 0.25 (0.5–1.3) cm in SCCd, and 1.21 ± 0.43 (0.8–2) cm in AW. Regarding symptoms in this group, one pineal cyst was incidentally found, and the other two had minor headache. Three patients had depression, numbness in the lower extremities, and visual disturbances with dysphasia and vertigo, associated with headache. All the rest had moderate to severe headache. Patients of this group were followed every 6 months/1 year. The average time between the initial diagnosis and the surgical date was 3.3 ± 2.32 (0.5–7) years.
Six patients met indication criteria for surgery during the past 5 years of the study with an average observation time before surgery of 4 ± 2.4 (1–7) years. The four cases that were operated earlier than 2011 had an average observation period before operation of 2.1 ± 2 (0.5–5) years. This finding reflected the progressively more conservative management of enlarging pineal cysts over the years.
Criterion 4 – 24 of the 60 pineal cysts presented with a solid component. The decision to proceed with surgery was based on a careful case by case evaluation by neurosurgeons and neuroradiologists. 13 (54%) patients with symptoms such as persistent headache, vertigo, dysesthesia, insomnia, changes in the personality, nausea, lack of speech development, galactorrhea, and visual disturbances had a radiographic evaluation concerning for an underlying neoplasm of unclear pathology, and they were operated on mainly due to this reason. The average size of these pineal lesions was 1.62 ± 0.36 (1.1–2.3) cm in SAPd, 1.10 ± 0.26 (0.6–1.66) cm in SCCd, and 1.32 ± 0.30 (0.7–1.93) cm in AW. Details about the solid components may be found in the radiological features section and the discussion.
Criterion 5 – 19 patients harboring large cysts presented with rather unspecific symptoms as follows: headache, not well defined visual disturbances, depression, psychosis, disorientation, nausea, memory deficits, balance instability, vertigo, episodic loss of consciousness, exertional headache, periorbital pain, tremor in a 8-year-old patient, and corporal or facial sensory deficits. Some symptoms without correlation to the disease such as epilepsy, hemifacial edema, tinnitus, cervicalgia, and sarcoidosis, were also encountered. The average pineal cyst size in this group was 2.13 ± 0.37 (1.6–3.1) cm in SAPd, 1.41 ± 0.32 (0.9–2) cm in SCCd, and 1.56 ± 0.41 (0.8–2.3) cm in AW.
Ten patients did not display any other criterion. Seven patients with very disabling symptoms had cyst diameters smaller than 20 mm: in 4 cases, the maximum diameter was 18 mm, 19 mm in 2, and 16 mm in one case.
Surgical treatment
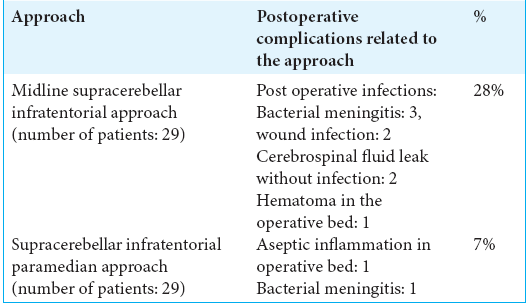
Gross total removal was achieved in 58 cases. A patient had a small residual cystic remnant that after few weeks compressed and occluded the aqueduct below the tectal plate and required an endoscopic third ventriculostomy, and one other pineal cyst was punctured by a stereotactic procedure with the aspiration of the cyst content without postoperative complications. Most patients were operated on by the senior author (JH). Three patients were operated on by other surgeons, including the one case of the stereotactic procedure in the supine position as a less invasive technique instead of the more demanding microsurgical approach. Two of these cases followed a paramedian supracerebellar infratentorial approach in a sitting position, and a suboccipital transtentorial approach. The supracerebellar infratentorial approach in the “sitting praying position” was chosen as the standard approach.
Before 2008, a straight midline approach was almost always used over the current paramedian supracerebellar route.[
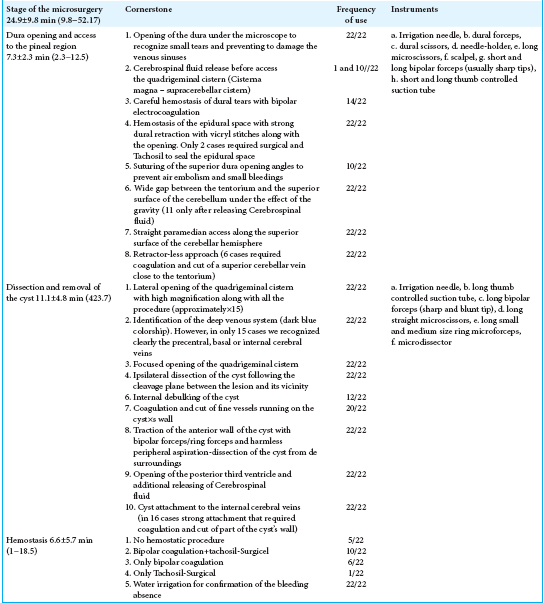
Based on the analysis of several surgical videos, we were able to determine some key steps in the surgical resection of pineal cysts as described in
Pathophysiology
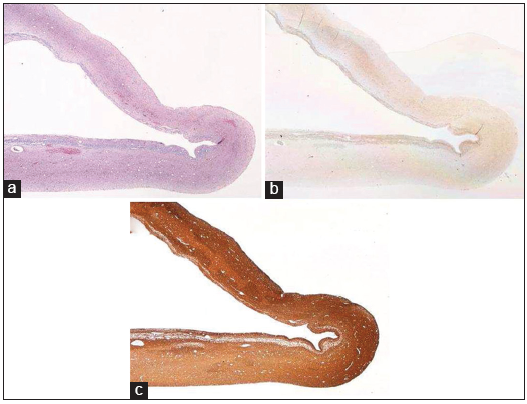
All cases in our cohort had histological confirmation of benign pineal cysts (WHO I) by an experienced pathologist. As reported in literature, histologic features were characteristic for inner glial tissue surrounded by outer pineal gland tissue. The cyst cavity was usually unilocular or multilocular without epithelial lining. Fibrillary astrocytes were immunopositive for antibody stains to glial fibrillary acidic protein (GFAP) and S100 protein. The peripheral pineal cells were positive to synaptophysin and neurofilament protein. A small interstitial component of the pineal cells was positive for GFAP and S-100 protein.[
Radiological characteristics
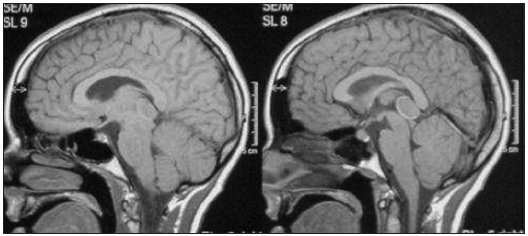
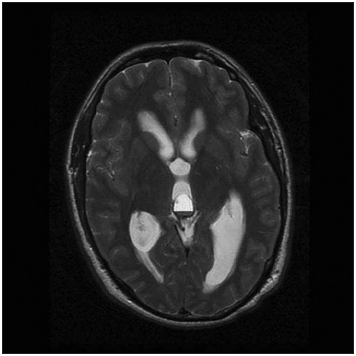
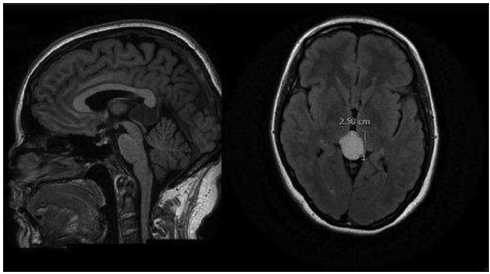
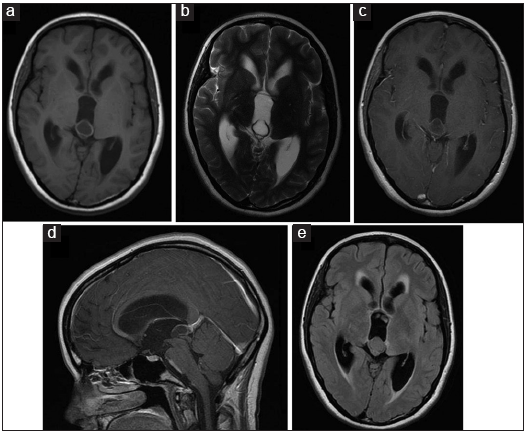
A CT scan was usually the first examination performed in head injured patients and acute hydrocephalus cases. Nevertheless, MRI remains our gold standard for both initial assessment and follow-up imaging. MRI was available in 56 cases. Most of the pineal cysts had a typical MRI appearance of a well-defined T1-hyperintense and T2-isointense round-shaped lesion, with homogenous interior signal characteristics, a rim of contrast-enhancement with <2 mm wall thickness, and no infiltration of the surrounding structures [
Figure 7
Classic MRI appearance of a benign pineal cyst. (a) T1-hyperintense lesion compared to CSF in axial view, (b) T2-isointense lesion compared to CSF in axial view, (c) ring enhancement lesion after intravenous contrast administration in T1 axial view, (d) ring enhancement lesion after intravenous contrast administration in T1 sagittal view, and (e) isointense lesion compared to brain tissue in FLAIR axial view.
As mentioned, 24 lesions showed some solid component among small thickenings of the cystic wall and large fragments of the eccentrically solid portions. The solid components were also characterized by irregular inner limits – one of them even confused as a parasitic lesion – with usually consistent enhancement after intravenous contrast administration. Intracystic septations were also visible in 11 cases, and three patients presented fluid-fluid level on the axial T2 sequence signing the pineal apoplexy.
Outcome
Clinical outcome
All patients except one improved clinically at last follow-up with most showing a complete recovery. All patients except one were functionally independent postoperatively. The one impaired patient remaining unfit to carry out activities due to a persistent though improved memory deficit and slight right-hand clumsiness (modified Rankin Scale 2). No other persistent perioperative complications were encountered. However, during an early postoperative assessment, three Parinaud’s syndromes were observed in our patients. Two of them resolved completely, while the third one improved substantially with very minimal residual double vision present at last evaluation 7 months after surgery.
Complications such as postoperative bacterial meningitis (4), wound infection without meningitis (2), and CSF leak without infection (2) were observed in 8 of our patients, with the last case presented in 2005. All cases were successfully managed with antibiotics, though two of them required reoperation. The long-term evaluation of our patients revealed no significant sequelae with only occasional headaches in a few of them.
Radiological outcome
One patient with a small hematoma in the operative bed was managed conservatively. She initially presented with new-onset postoperative visual disturbances and depression, which progressively resolved with the resorption of the hematoma. A second patient developed an aseptic granulomatous reaction following an open surgical biopsy. For this, she was treated medically and continued to follow-up.
DISCUSSION
In this paper, we present our microsurgical management of 60 consecutive patients presenting with benign pineal cysts. We assessed our indications and categorized them into specific criteria for pineal cysts surgery, and we evaluated the surgical outcome, and we analyzed the key steps for microsurgery.
As concluded by some authors, despite the advances in high-resolution MRI, there are not definite radiological methods to distinguish benign pineal cysts from pineal region malignancies containing cystic components such as pineocytomas, pineoblastomas, germinomas, or mature teratomas.[
Inclusion criteria for pineal cyst neurosurgery
The symptoms in our surgically treated pineal cyst patients do not differ from those reported in other series: headache being the most common symptom (with or without hydrocephalus) besides other nonspecific symptoms such as numbness, visual disturbances, vertigo, balance disturbances, and memory deficits. Although some symptoms might be clearly explained by hydrocephalus or the compressive effect of the tectum, the association between the symptoms and the cyst becomes hard to define in the absence of ventriculomegaly or Parinaud’s syndrome. Radoš et al. hypothesize that CSF can be permanently produced and absorbed inside the brain ventricles, as well as inside the entire CSF system, as a consequence of water filtration and reabsorption through the capillary walls into the interstitial fluid of the surrounding central nervous system tissue. Thus, the obstruction of the aqueduct of Sylvius cannot be the only cause of hydrocephalus development, but the association with other pathological processes that impair filtration and reabsorption of fluids on the capillary level such as bleeding, infection, tumor, and toxic substances, among others.[
Another indication for pineal cyst surgery was the presence of nonspecific symptoms in the context of a radiographically detected lesion with a solid component. It is well known that pineal cysts frequently contain solid structures and these may even enhance after contrast injection.[
During the past years, evidence about the natural history of this benign lesion, which includes spontaneous shrinkage, have led to the development of more stringent indications for the surgical treatment of pineal cysts.[
Interestingly, our results showed that the mean pineal cyst diameters in patients with clinical and radiographic hydrocephalus were 2.5 ± 0.96 (SAPd) × 1.6 ± 0.85 (SCCd) × 1.9 ± 0.48 (AW) cm. On the other hand, large cysts with unspecific symptoms had a mean size of 2.1 ± 0.37 (SAPd) × 1.4 ± 0.32 (SCCd) × 1.6 ± 0.41 (AW) cm and pineal cysts – in patients harboring mainly headache – that increased in size along the years – up to 1.9 ± 0.30 (SAPd) × 1.3 ± 0.38 (SCCd) × 1.5 ± 0.45 (AW) – measured initially 1.6 ± 0.26 (SAPd) × 1.1 ± 0.25 (SCCd) × 1.2 ± 0.43 (AW).
In this regard, our findings might suggest that pineal cysts have progressive clinical evolution with obstructive hydrocephalus at the latest stage in acute or progressive diseases, with a mean cysts size of 2.5 cm. However, cysts with minimal symptoms such as headache and those cysts that will probably require surgery during the follow up period might initially present with a mean SAPd of only 1.6 cm (1.2 cm at minimum). In contrast to Al-Holou et al., who had in their extensive series a large number of pineal cysts that decreased in size at the follow-up, we hypothesize that once a minimally symptomatic cyst is equal or larger than 1.5 cm, the risk to increase in size, worsening the symptoms, and requiring surgery in the future is high, particularly in people with ages ranging between 15 and 40 years old.[
Furthermore, we noted that more conservative management has been adopted in our department over recent years for pineal cysts that are not clearly symptomatic, even if they were growing during the follow-up period. However, we continue to believe that symptomatic lesions clearly meet indication criteria for surgery, and especially tumors with mixed radiographic components should be carefully evaluated in cooperation with the neuroradiologists.
Surgical outcome
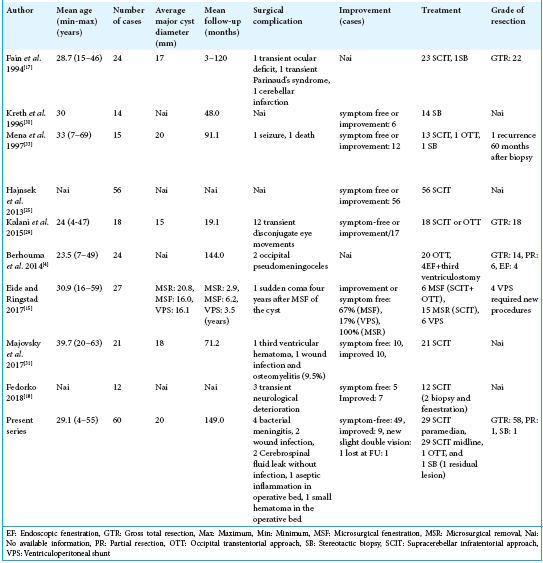
Our cohort study is the largest reported series of surgically treated pineal cysts thus far.[
Pitfalls in pineal cyst microsurgery
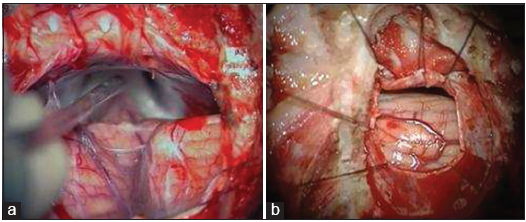
The rather short surgical and microsurgical time of our operative procedures reflect the efficiency of our operative technique. The paramedian supracerebellar infratentorial approach in a sitting position is a standard procedure that was perfected over many the years by the senior author (JH). Th e key steps of this particular approach are described in detail in previous papers.[
The pivotal microsurgical steps may be divided into three groups:
The dura opening and access to the pineal region The microsurgical removal of the cyst The hemostasis.
All the microsurgical features were meticulously reviewed and optimized according to these three steps. Other authors have also presented general data from their retrospective reviews of traditionally written surgical reports. However, we believe that a careful analysis and review of surgical videos on the topic may reveal some pertinent aspect of the technique that escaped prior reporting. By submitting a link to our surgical recording (https://surgicalneurologyint.com/videogallery/pineal-cyst/), we this hope to better describe the different steps of the microsurgical removal of pineal cysts.
Future perspectives
This is a retrospective study based on the experience of a single institution over the past 20 years; however, new protocols and new approaches are continuously evolving. Endoscopic procedures have been reported in the more recent literature for the management of pineal cysts.[
Similarly, a variety of stereotactic procedures have been reported.[
Our results from this large series demonstrate how the microsurgical management performed particularly under the small paramedian – instead of a midline – supracerebellar infratentorial approach in a sitting position, is currently our most effective and safest approach symptomatic pineal cysts. Thus, virtually atraumatic procedures with small craniotomies and small dura openings can yield a reduction of postoperative complications. Needless to say, the development of excellent microsurgical skills is required for dealing with these deep-seated lesions.
CONCLUSION
We describe in this paper the largest series of microsurgically treated pineal cysts and report excellent clinical outcomes with our approach. A judicious microsurgical technique is in our eyes the most suitable technique to effectively deal with these benign lesions in a complex location.
Financial support and sponsorship
Ehrnrooth foundation supported the present manuscript.
Conflicts of interest
There are no conflicts of interest.
References
1. Al-Holou WN, Terman SW, Kilburg C, Garton HJ, Muraszko KM, Chandler WF. Prevalence and natural history of pineal cysts in adults. J Neurosurg. 2011. 115: 1106-14
2. Barat JL, Benabid A, Blond S, Brunon J, Chazal J, Cohadon F. Stereotaxic biopsies of pineal tumors. Comments on their risk and implication apropos of 370 cases. Neurochirurgie. 1994. 40: 3-9
3. Barboriak DP, Lee L, Provenzale JM. Serial MR imaging of pineal cysts: Implications for natural history and follow-up. AJR Am J Roentgenol. 2001. 176: 737-743
4. Berhouma M, Ni H, Delabar V, Tahhan N, Salem SM, Mottolese C. Update on the management of pineal cysts: Case series and a review of the literature. Neurochirurgie. 2015. 61: 201-7
5. Bezuidenhout AF, Kasper EM, Baledent O, Rojas R, Bhadelia RA. Relationship between pineal cyst size and aqueductal CSF flow measured by phase contrast MRI. J Neurosurg Sci. 2018. p.
6. Carr J. Cystic hydrops of the pineal gland. J Nerv Dis. 1944. 99: 552-72
7. Cauley KA, Linnell GJ, Braff SP, Filippi CG. Serial follow-up MRI of indeterminate cystic lesions of the pineal region: Experience at a rural tertiary care referral center. AJR Am J Roentgenol. 2009. 193: 533-7
8. Chiechi MV, Smirniotopoulos JG, Mena H. Pineal parenchymal tumors: CT and MR features. J Comput Assist Tomogr. 1995. 19: 509-17
9. Choque-Velasquez J, Colasanti R, Resendiz-Nieves JC, Gonzáles-Echevarría KE, Raj R, Jahromi BR. Praying sitting position for pineal region surgery: An efficient variant of a classic position in neurosurgery. World Neurosurg. 2018. 113: e604-11
10. Choque-Velasquez J, Colasanti R, Resendiz-Nieves JC, Jahromi BR, Kozyrev DA, Thiarawat P. Supracerebellar infratentorial paramedian approach in helsinki neurosurgery: Cornerstones of a safe and effective route to the pineal region. World Neurosurg. 2017. 105: 534-42
11. Choque-Velasquez J, Colasanti R, Resendiz-Nieves JC, Raj R, Lindroos AC, Jahromi BR. Venous air embolisms and sitting position in Helsinki pineal region surgery. Surg Neurol Int. 2018. 9: 160-
12. Choque-Velasquez J, Miranda-Solis F, Colasanti R, Ccahuantico-Choquevilca LA, Hernesniemi J. Modified pure endoscopic approach to the pineal region: A proof of concept of an efficient and inexpensive surgical model based on laboratory dissections. World Neurosurg. 2018. 117: 195-8
13. de Jong MC, Moll AC, Göricke S, van der Valk P, Kors WA, Castelijns JA. From a suspicious cystic pineal gland to pineoblastoma in a patient with familial unilateral retinoblastoma. Ophthalmic Genet. 2016. 37: 116-8
14. Eide PK, Ringstad G. Increased pulsatile intracranial pressure in patients with symptomatic pineal cysts and magnetic resonance imaging biomarkers indicative of central venous hypertension. J Neurol Sci. 2016. 367: 247-55
15. Eide PK, Ringstad G. Results of surgery in symptomatic non-hydrocephalic pineal cysts: Role of magnetic resonance imaging biomarkers indicative of central venous hypertension. Acta Neurochir (Wien). 2017. 159: 349-61
16. Engel U, Gottschalk S, Niehaus L, Lehmann R, May C, Vogel S. Cystic lesions of the pineal region--MRI and pathology. Neuroradiology. 2000. 42: 399-402
17. Fain JS, Tomlinson FH, Scheithauer BW, Parisi JE, Fletcher GP, Kelly PJ. Symptomatic glial cysts of the pineal gland. J Neurosurg. 1994. 80: 454-60
18. Fedorko S, Zweckberger K, Unterberg AW. Quality of life following surgical treatment of lesions within the pineal region. J Neurosurg. 2018. 130: 1-10
19. Fetell MR, Bruce JN, Burke AM, Cross DT, Torres RA, Powers JM. Non-neoplastic pineal cysts. Neurology. 1991. 41: 1034-40
20. Gaab MR, Schroeder HW. Neuroendoscopic approach to intraventricular lesions. J Neurosurg. 1998. 88: 496-505
21. Gokce E, Beyhan M. Evaluation of pineal cysts with magnetic resonance imaging. World J Radiol. 2018. 10: 65-77
22. Golzarian J, Balériaux D, Bank WO, Matos C, Flament-Durand J. Pineal cyst: Normal or pathological?. Neuroradiology. 1993. 35: 251-3
23. Gore PA, Gonzalez LF, Rekate HL, Nakaji P. Endoscopic supracerebellar infratentorial approach for pineal cyst resection: Technical case report. Neurosurgery. 2008. 62: 108-9
24. Griffith HB. Technique of fontanelle and persutural ventriculoscopy and endoscopic ventricular surgery in infants. Childs Brain. 1975. 1: 359-63
25. Hajnsek S, Paladino J, Gadze ZP, Nanković S, Mrak G, Lupret V. Clinical and neurophysiological changes in patients with pineal region expansions. Coll Antropol. 2013. 37: 35-40
26. Hellwig D, Bauer BL, List-Hellwig E. Stereotactic endoscopic interventions in cystic brain lesions. Acta Neurochir Suppl. 1995. 64: 59-63
27. Hernesniemi J, Romani R, Albayrak BS, Lehto H, Dashti R, Ramsey C. Microsurgical management of pineal region lesions: Personal experience with 119 patients. Surg Neurol. 2008. 70: 576-83
28. Husain N, Kumari M, Husain M. Tumor irrigation fluid enhances diagnostic efficacy in endoscopic biopsies of intracranial space-occupying lesions. Acta Neurochir (Wien). 2010. 152: 111-7
29. Kalani MY, Wilson DA, Koechlin NO, Abuhusain HJ, Dlouhy BJ, Gunawardena MP. Pineal cyst resection in the absence of ventriculomegaly or Parinaud’s syndrome: Clinical outcomes and implications for patient selection. J Neurosurg. 2015. 123: 352-6
30. Kreth FW, Schätz CR, Pagenstecher A, Faist M, Volk B, Ostertag CB. Stereotactic management of lesions of the pineal region. Neurosurgery. 1996. 39: 280-9
31. Májovský M, Netuka D, Beneš V. Conservative and surgical treatment of patients with pineal cysts: Prospective case series of 110 patients. World Neurosurg. 2017. 105: 199-205
32. Mamourian AC, Towfighi J. Pineal cysts: MR imaging. AJNR Am J Neuroradiol. 1986. 7: 1081-6
33. Mena H, Armonda RA, Ribas JL, Ondra SL, Rushing EJ. Nonneoplastic pineal cysts: A clinicopathologic study of twenty-one cases. Ann Diagn Pathol. 1997. 1: 11-8
34. Michielsen G, Benoit Y, Baert E, Meire F, Caemaert J. Symptomatic pineal cysts: Clinical manifestations and management. Acta Neurochir (Wien). 2002. 144: 233-42
35. Musolino A, Cambria S, Rizzo G, Cambria M. Symptomatic cysts of the pineal gland: Stereotactic diagnosis and treatment of two cases and review of the literature. Neurosurgery. 1993. 32: 315-20
36. Nakazato Y, Jouvet A, Scheithauer BW.editors. Pineoblastoma. WHO Classification of Tumours of the Central Nervous System. Lyon: International Agency for Research on Cancer (IARC); 2007. p. 126-7
37. Radoš M, Orešković D, Radoš M, Jurjević I, Klarica M. Long lasting near-obstruction stenosis of mesencephalic aqueduct without development of hydrocephalus--case report. Croat Med J. 2014. 55: 394-8
38. Starke RM, Cappuzzo JM, Erickson NJ, Sherman JH. Pineal cysts and other pineal region malignancies: Determining factors predictive of hydrocephalus and malignancy. J Neurosurg. 2017. 127: 249-54
39. Stern JD, Ross DA. Stereotactic management of benign pineal region cysts: Report of two cases. Neurosurgery. 1993. 32: 310-4
40. Sugiyama K, Arita K, Okamura T, Yamasaki F, Kajiwara Y, Ueda H. Detection of a pineoblastoma with large central cyst in a young child. Childs Nerv Syst ChNS. 2002. 18: 157-60
41. Thaher F, Kurucz P, Fuellbier L, Bittl M, Hopf NJ. Endoscopic surgery for tumors of the pineal region via a paramedian infratentorial supracerebellar keyhole approach (PISKA). Neurosurg Rev. 2014. 37: 677-84
42. Turtz AR, Hughes WB, Goldman HW. Endoscopic treatment of a symptomatic pineal cyst: Technical case report. Neurosurgery. 1995. 37: 1013-4
43. Uschold T, Abla AA, Fusco D, Bristol RE, Nakaji P. Supracerebellar infratentorial endoscopically controlled resection of pineal lesions: Case series and operative technique. J Neurosurg Pediatr. 2011. 8: 554-64
44. Welton PL, Reicher MA, Kellerhouse LE, Ott KH. MR of benign pineal cyst. AJNR Am J Neuroradiol. 1988. 9: 612-






Anna A Halfhill
Posted November 15, 2019, 12:28 am
Is there anybody that can help me. I live in Fairbanks Alaska I have a symptomatic cyst I had a fenestration 3 years ago when I had private insurance. Since then my cyst has closed back off and has been very slowly growing. I have tried everything to get a neurosurgeon to operate to have a shunt placed but I now have Medicaid and Medicare.