- Department of Neurosurgery, Princess Alexandra Hospital, Brisbane, Australia.
Correspondence Address:
Ning Zhu, Department of Neurosurgery, Princess Alexandra Hospital, Brisbane, Australia.
DOI:10.25259/SNI_293_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ning Zhu, Ananthababu Pattavilakom Sadasivan. The need for an institution-specific normal pressure hydrocephalus management protocol. 03-Jun-2022;13:236
How to cite this URL: Ning Zhu, Ananthababu Pattavilakom Sadasivan. The need for an institution-specific normal pressure hydrocephalus management protocol. 03-Jun-2022;13:236. Available from: https://surgicalneurologyint.com/surgicalint-articles/11631/
Abstract
Background: Despite the publication of international guidelines, the management of normal pressure hydrocephalus (NPH) varies due to clinician preference and varying clinical evidence. An audit was performed to review the current pathways used in clinical practice with the aim of formulating an institution-specific protocol to optimize and standardize care.
Methods: An internal audit was performed on the management of patients with NPH who presented to the Princess Alexandra Hospital, Brisbane between January 2016 and February 2019.
Results: Forty-one patients were included in the study. Lumbar puncture (LP) was the main diagnostic test used (63.4%). About 14.6% underwent lumbar drain (LD) insertion instead. About 12.2% did not undergo either LP or LD before definitive treatment. Only 60% of all patients underwent ventriculoperitoneal shunt insertion. Overall, five treatment pathways were noted. LP + VP shunt showed the greatest average improvement in Montreal Cognitive Assessment (MoCA) or Mini-Mental State Examination (MMSE) (+3.8 ± 3.18), followed by LD + VP shunt (+3.25 ± 3.52) and sole treatment with LP (+1.83 ± 1.18). Both pre and post intervention assessment of gait and cognition were only performed in 31% and 48.8% of patients, respectively. Four types of cognitive assessment were used (MOCA 46.4%, MMSE 46.4%, Rowland Universal Dementia Assessment Scale 3.6%, and Addenbrooke’s Cognitive Examination-III 3.6%). MoCA showed greater cognition improvement (2.07) compared to MMSE (1.3) post intervention. There was no consistent objective gait assessment test used.
Conclusion: The multiple NPH treatment pathways, low rate of pre and post objective symptom assessment, and lack of standardized gait and cognitive assessment tests demonstrate a clear need for an institution-specific NPH management protocol to standardize diagnostic workup, definitive management, and allied health assessment.
Keywords: Diagnosis, Normal pressure hydrocephalus, Protocol, Treatment
INTRODUCTION
Normal pressure hydrocephalus (NPH) is characterized by a triad of gait disturbance, cognitive impairment, and urinary dysfunction. This is usually associated with ventriculomegaly and normal CSF pressure.[
NPH did not have published management guidelines until recent years.[
The effectiveness of a confirmatory test or definitive treatment is usually assessed by subjective and objective improvements in gait and cognitive impairment.
Gait disturbance can manifest as an ataxic wide-based gait or a short stepped, shuffling gait.[
Cognitive impairment in NPH is characterized by frontosubcortical dysfunction. This manifests as psychomotor slowing as well as impaired attention, short-term memory, and executive function.[
The purpose of this study is to review the diagnostic and management pathways of NPH within an Australian tertiary hospital and to formulate an institution-specific protocol to standardize care.
MATERIALS AND METHODS
This study was a retrospective analysis of the diagnostic and management pathways of patients with NPH who presented to the Princess Alexandra Hospital (Brisbane, Australia) between January 2016 and February 2019.
Patients were included if they had a confirmed diagnosis of NPH and were investigated or treated for NPH at PAH between January 1, 2016, and February 30, 2019.
Notes containing each patient’s presenting symptoms, imaging reports, diagnostic method/confirmatory tests, and definitive treatment were manually extracted from the hospital’s electronic medical records system (iEMR) and the state-based electronic records system “The Viewer” through discharge summaries and correspondence from GPs and private specialists.
RESULTS
Forty-one patients underwent diagnosis and/or treatment for NPH at the Princess Alexandra Hospital between January 2016 and February 2019. The ages ranged from 55 to 91 with the average age being 73. The average age of NPH diagnosis was 70 years. The ratio of males-to-females was 29:16.
Overall, 63.4% had a LP as a part of their care, compared to 14.6% who had a lumbar drain. About 12.2% did not undergo either LP or LD before definitive treatment. About 57.7% of those who underwent LP went on to have ventriculoperitoneal shunt inserted. About 83% of those who underwent LD went on to have a ventriculoperitoneal shunt inserted. About 60% of all patients had a ventriculoperitoneal shunt inserted.
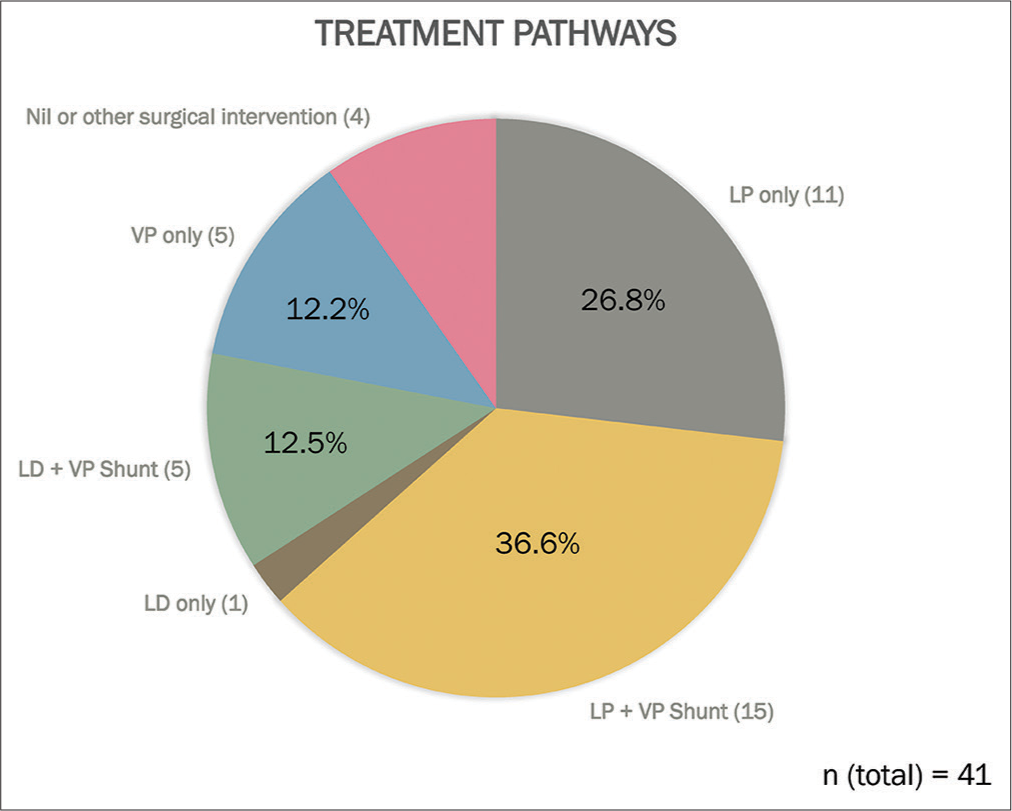
Overall, five main treatment pathways were noted: LP followed by VP shunt (36.6%); LP only (26.8%); VP shunt only (12.2%); LD followed by a VP shunt (12.2%); and LD only (2.4%). About 4.8% underwent other surgical interventions and another 4.8% patients refused treatment [
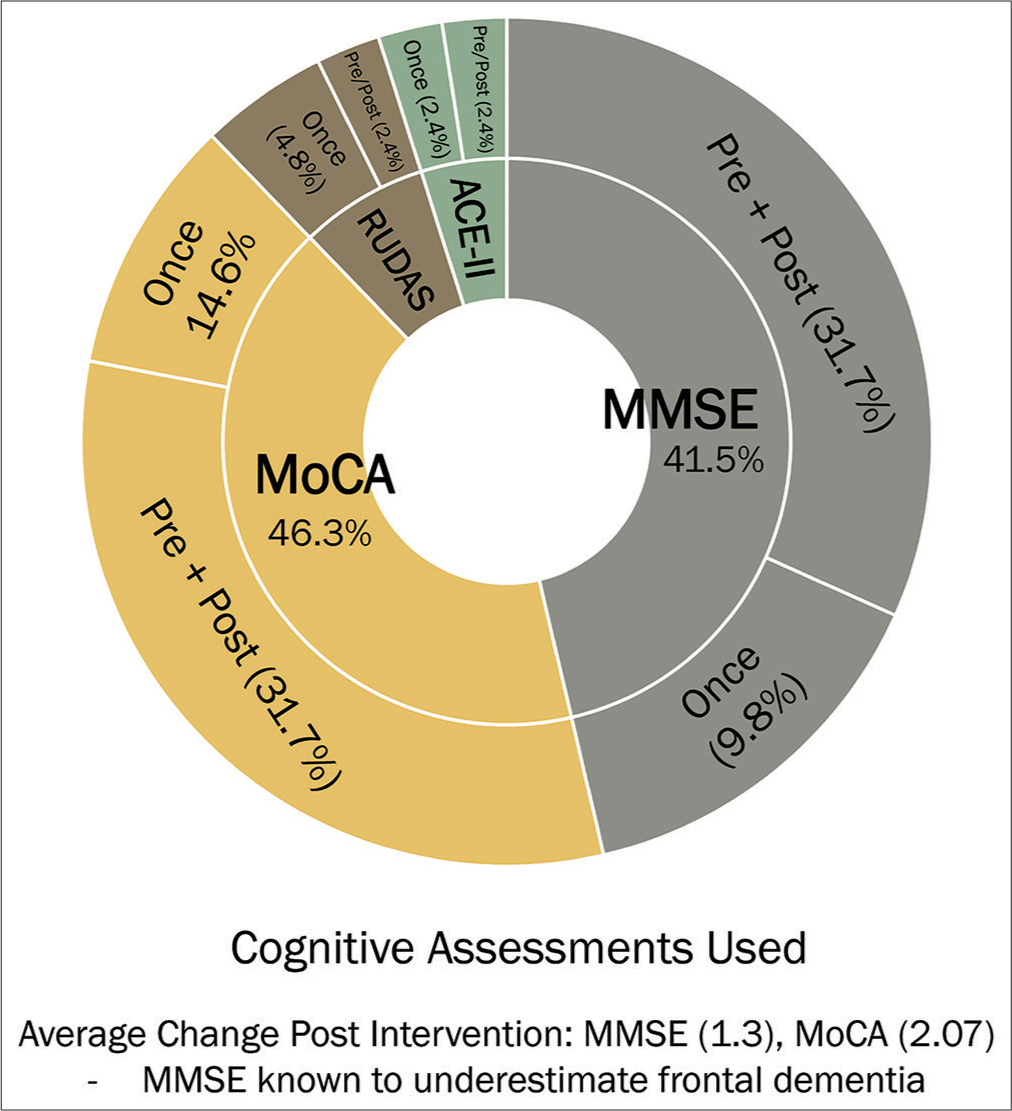
Four types of cognitive assessment were used: MMSE, MoCA, Rowland Universal Dementia Assessment Scale (RUDAS), and the Addenbrooke’s Cognitive Examination-III [
Figure 2:
Distribution of cognitive assessments, there were four cognitive assessment tests used: Montreal Cognitive Assessment (MoCA, 46.3%), Mini-Mental Status Examination (MMSE, 41.5%), Rowland Universal Dementia Assessment Scale (RUDAS, 7.2%), and the Addenbrooke’s Cognitive Examination III (ACE-III, 4.8%).
Despite 73.1% undergoing cognitive assessment at some point, however, only 48.8% were assessed both pre and post intervention. In those who underwent both pre and post intervention assessment, MOCA and MMSE were equally used (46.4%), while RUDAS and ACE-II were each used in 3.6% of patients.
Only 31% of patients underwent an objective gait assessment (e.g., TUG) despite 80% being assessed by a physiotherapist.
Cognitive and gait assessment were performed at variable times pre and post intervention and only when patients were admitted as an inpatient. These were not performed on outpatient follow-up.
Only 50% who underwent LP had their opening pressures recorded, while the average volume of CSF removed was 30 ml.
MoCA showed a greater average score change post intervention than MMSE with changes of 2.07 ± 1.38 and 1.30 ± 1.14 out of 30, respectively. The average change following LP was also +2.63 ± 2.09 (MoCA) and +1.2 ± 0.96 (MMSE), while the average change following a VP shunt was +1.55 ± 1.26 (MoCA) and +0.25 ± 1.68 (MMSE).
Lumbar drain insertion showed a greater increase in MMSE score (+2.5 ± 2.29) compared to LP (+1.2 ± 1.09).
Regarding treatment pathways, those who only underwent a LP showed an average improvement in cognitive assessment score (in either MoCA or MMSE) of +1.83 ± 1.18 out of 30; while having a lumbar drain followed by a VP shunt showed a change of +3.25 ± 3.52. Patients who underwent a LP followed by a VP shunt showed the greatest average cognitive score change of +3.8 ± 3.18. Cognitive assessment for patients who only underwent a VP shunt was not performed.
The average improvement in TUG was 4.77s ± 5.57 following an LP, 3.37s ± 9.38 following a lumbar drain, and 3.75s ± 4.56 following any intervention.
DISCUSSION
This study allowed for an audit of the clinically applied management pathways of NPH in an Australian tertiary hospital.
For diagnosis, LP was the main confirmatory test used (63.4%). This was likely due to several factors: more widespread skill competence among medical staff, shorter inpatient stays, and reduced risk of adverse effects. LP has a sensitivity of 58% and specificity of 75% and is the recommended CSF drainage test.[
42.3% of those who underwent LP did not proceed with definitive management. This was likely due to inadequate improvement to justify surgical intervention or patient objection to surgery. A higher proportion of patients who had a lumbar drain inserted (83%) proceeded to insertion of a ventriculoperitoneal shunt compared to those who had a LP (57.7%). This could be attributed to greater volume of diverted CSF allowed by a lumbar drain (~10 ml/h) over 72 h compared a single reduction in CSF volume of 30 ml in LP.
12.2% of all patients did not undergo a confirmatory test and proceeded to ventriculoperitoneal shunt insertion. While the correlation of symptoms and radiological findings can provide strong diagnostic evidence for NPH, the foregoing of a confirmatory CSF drainage test can risk insertion of a VP shunt in a patient with no subsequent clinical improvement.
Five treatment pathways were likely due to varying clinical evidence and individual clinician preference. The most used pathway (36.6%) was diagnosis with a LP, followed by definitive management by insertion of a ventriculoperitoneal shunt. This is the pathway that is most recommended by the latest published guidelines by Nakajima et al.[
There was a low rate of pre and post intervention objective assessment of NPH symptoms by allied health staff. Only 48.8% underwent pre and post cognitive assessment, while only 31% underwent pre and post objective gait assessment. This was likely due to the lack of a management protocol mandating assessment pre and post intervention at our institution as well as inadequate allied health staffing to facilitate this service when requested by the medical team.
The clear need for an institution-specific NPH management protocol was shown by the multiple treatment pathways, the low rate of pre and post objective symptom assessment and the lack of standardized gait and cognitive assessment tests.
It must be stated that our study had a small patient population and low pre and post intervention symptom assessment rates. As such, our single institution study had very limited study power. This is demonstrated by our statistically nonsignificant findings. Nevertheless, our findings were consistent with known guidelines. Regarding cognitive assessment test, MOCA and MMSE were used equally. Our study suggested that MoCA was possibly more sensitive than MMSE, which is consistent with the previous evidence that MMSE underestimates frontal dementia when compared to other assessment tests.[
Interestingly, lumbar drain insertion was suggested to show greater improvement in MMSE score than LP. Regarding gait assessment, TUG was generally used when objective assessment was undertaken. There was insufficient and inconsistent assessment of gait to provide a comparison between tests; however, the literature and current guidelines suggest the use of a timed 10 m straight walking test and a TUG.[
In future studies with a multi-institution study with a larger study population, a proper analysis of treatment pathways and cognitive assessment tests would be possible.
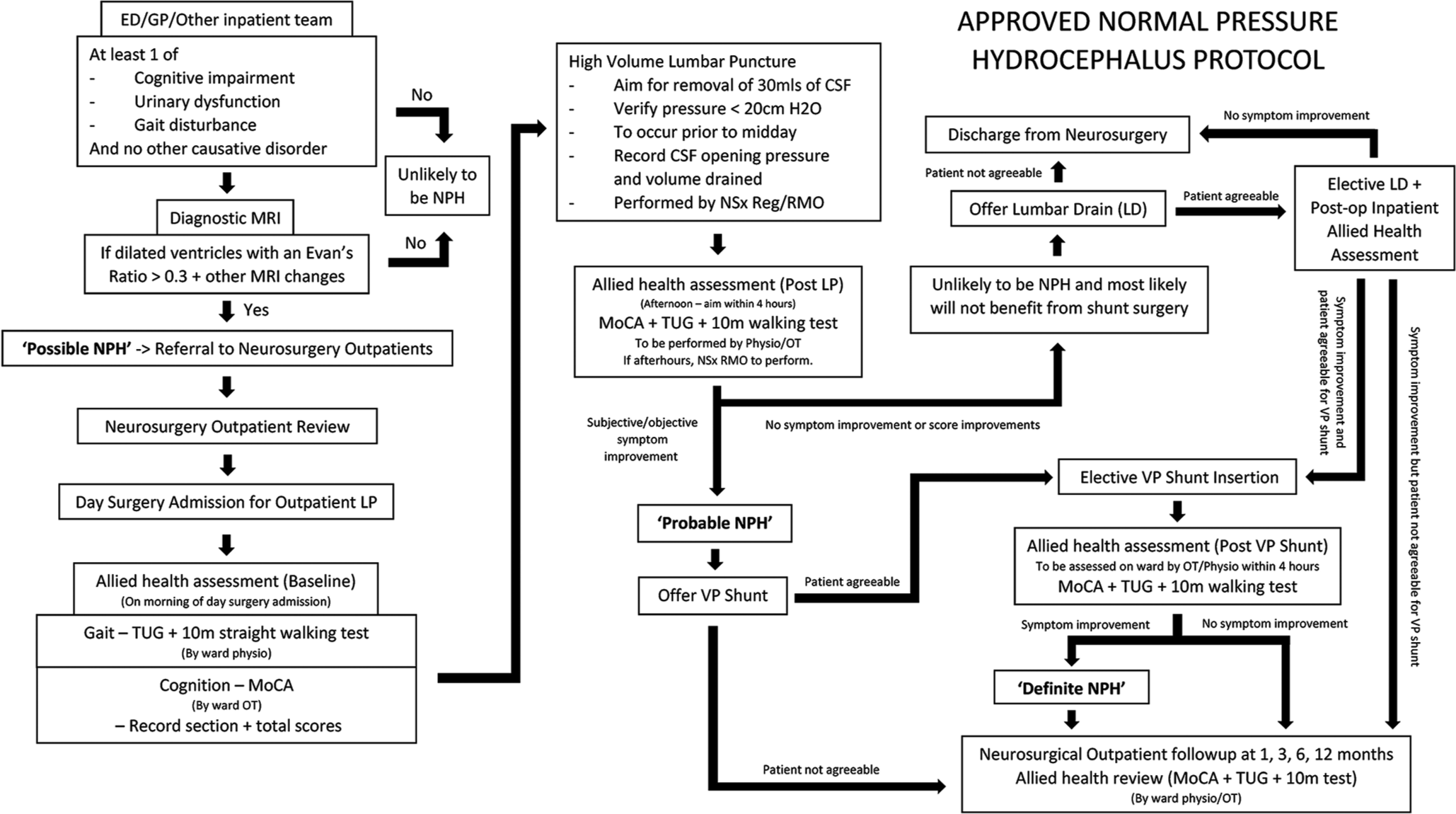
Following consideration of the current published guidelines, local resources, and discussion with relevant allied health and medical staff, we created a hospital-specific NPH management protocol [
CONCLUSION
In our hospital, there are multiple NPH treatment pathways, a low rate of pre and post objective symptom assessment and a lack of standardized gait and cognitive assessment tests. We created a hospital-specific NPH management protocol to standardize care at our institution. Given our findings, we suggest that other institutions also review their current practices to optimize and standardize NPH management. With further research across multiple institutions and with a larger study population, statistically significant findings when comparing individual assessment and interventions are likely and an externally valid protocol could be created.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
Many thanks to Dr. Damian Amato and Dr. Sarah Olson and Shari Canagasuriam (Occupational therapist) and Treena Seeto (Physiotherapist) for providing valuable insight and recommendations for the creation of the NPH protocol.
References
1. Bergsneider M, Black PM, Klinge P, Marmarou A, Relkin N. Surgical management of idiopathic normal-pressure hydrocephalus. Neurosurgery. 2005. 57: S29-39
2. Bräutigam K, Vakis A, Tsitsipanis C. pathogenesis of idiopathic normal pressure hydrocephalus: A review of knowledge. J Clin Neurosci. 2019. 61: 10-3
3. Fleming WB, Xerri J, Tailor P, Raman A. Proposed guidelines for the assessment and management of idiopathic normal pressure hydrocephalus in the United Kingdom. J Neurol Neurosurg Psychiatry. 2012. 83: A10
4. Graff-Radford N. Normal Pressure Hydrocephalus. Available from: https://www.uptodate.com/contents/normal-pressure-hydrocephalus [Last accessed 2019 Aug 06].
5. Ishikawa M. Clinical guidelines for idiopathic normal pressure hydrocephalus. Neurol Med Chir (Tokyo). 2004. 44: 222-3
6. Kiefer M, Unterberg A. The differential diagnosis and treatment of normal-pressure hydrocephalus. Dtsch Arztebl Int. 2012. 109: 15-25
7. Klinge P, Marmarou A, Bergsneider M, Relkin N, Black PM. Outcome of shunting in idiopathic normal-pressure hydrocephalus and the value of outcome assessment in shunted patients. Neurosurgery. 2005. 57: S40-52
8. Lucareli PB, Lacerda S, Hideyo I, Garbelotti S, Speciali D. Gait Deviation Index for the assessment of normal pressure hydrocephalus. Gait Posture. 2015. 42: S8
9. Marmarou A, Bergsneider M, Klinge P, Relkin N, Black PM. The value of supplemental prognostic tests for the preoperative assessment of idiopathic normal-pressure hydrocephalus. Neurosurgery. 2005. 57: S17-28
10. Marmarou A, Black P, Bergsneider M, Klinge P, Relkin N. Guidelines for management of idiopathic normal pressure hydrocephalus: Progress to date. Acta Neurochir Suppl. 2005. 95: 237-40
11. Miyoshi N, Kazui H, Ogino A, Ishikawa M, Miyake H, Tokunaga H. Association between cognitive impairment and gait disturbance in patients with idiopathic normal pressure hydrocephalus. Dement Geriatr Cogn Disord. 2005. 20: 71-6
12. Mori E, Ishikawa M, Kato T, Kazui H, Miyake H, Miyajima M.editors. Guidelines for management of idiopathic normal pressure hydrocephalus: Second edition. Neurol Med Chir (Tokyo). 2012. 52: 775-809
13. Nakajima M, Yamada S, Miyajima M, Ishii K, Kuriyama N, Kazui H.editors. Guidelines for management of idiopathic normal pressure hydrocephalus (third edition): Endorsed by the Japanese society of normal pressure hydrocephalus. Neurol Med Chir (Tokyo). 2021. 61: 63-97
14. Ogino A, Kazui H, Miyoshi N, Hashimoto M, Ohkawa S, Tokunaga H. Cognitive impairment in patients with idiopathic normal pressure hydrocephalus. Dement Geriatr Cogn Disord. 2006. 21: 113-9
15. Picascia M, Zangaglia R, Bernini S, Minafra B, Sinforiani E, Pacchetti C. A review of cognitive impairment and differential diagnosis in idiopathic normal pressure hydrocephalus. Funct Neurol. 2015. 30: 217-28
16. Relkin N, Marmarou A, Klinge P, Bergsneider M, Black PM. Diagnosing idiopathic normal-pressure hydrocephalus. Neurosurgery. 2005. 57: S4-16
17. Saito M, Nishio Y, Kanno S, Uchiyama M, Hayashi A, Takagi M. Cognitive profile of idiopathic normal pressure hydrocephalus. Dement Geriatr Cogn Dis Extra. 2011. 1: 202-11
18. Solana E, Sahuquillo J, Junqué C, Quintana M, Poca MA. Cognitive disturbances and neuropsychological changes after surgical treatment in a cohort of 185 patients with idiopathic normal pressure hydrocephalus. Arch Clin Neuropsychol. 2012. 27: 304-17
19. Tarnaris AW, Michael A. Idiopathic normal pressure hydrocephalus update and practical approach on diagnosis and management. Neurosurg Q. 2011. 21: 72-81
20. Vanneste JA. Diagnosis and management of normal-pressure hydrocephalus. J Neurol. 2000. 247: 5-14
21. Walchenbach R, Geiger E, Thomeer RT, Vanneste JA. The value of temporary external lumbar CSF drainage in predicting the outcome of shunting on normal pressure hydrocephalus. J Neurol Neurosurg Psychiatry. 2002. 72: 503-6