- Department of Neurosurgery, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh,
- Department of Vascular Surgery, Ibrahim Cardiac Hospital and Research Institute, Dhaka, Bangladesh,
- Department of Anaesthesia, Dreamzz IVF Centre and Women’s Care Hospital, Ahmedabad, Gujarat, India,
- Department of Neurosurgery, Bhawani Hospital and Research Centre, Birgunj, Nepal.
Correspondence Address:
Md. Rokibul Islam, Assistant Professor, Department of Neurosurgery, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh.
DOI:10.25259/SNI_967_2021
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Md. Rokibul Islam1, Ayub Ansari1, Asifur Rahman1, S. M. G. Saklayen2, Nur Muhammad1, Satish Kumar Shah1, Vishal K. Chavda3, Bipin Chaurasia4, Mohammad Hossain1. The perplexing postsurgical complication of carotid-jugular fistula: A bitter experience. 05-Jan-2022;13:2
How to cite this URL: Md. Rokibul Islam1, Ayub Ansari1, Asifur Rahman1, S. M. G. Saklayen2, Nur Muhammad1, Satish Kumar Shah1, Vishal K. Chavda3, Bipin Chaurasia4, Mohammad Hossain1. The perplexing postsurgical complication of carotid-jugular fistula: A bitter experience. 05-Jan-2022;13:2. Available from: https://surgicalneurologyint.com/surgicalint-articles/11332/
Abstract
Background: Vascular injuries occur in approximately 25% of all penetrating neck traumas, with carotid artery injuries being particularly lethal. Penetrating neck injuries are potentially fatal. Vascular injuries occur in approximately 25% of cases, which can lead to the formation of arteriovenous fistulas.
Case Description: The authors present a case of delayed open surgery to repair a carotid-jugular fistula that resulted in an unprecedented complication, as well as a brief review of the condition’s diagnosis and treatment options.
Conclusion: This case report suggests us that, penetrating neck injuries should be thoroughly evaluated for arteriovenous fistulae. To avoid complications, common carotid-jugular fistulas must be treated as soon as possible. Postoperative complications can be effectively managed with prompt action.
Keywords: Arteriovenous fistulas, Carotid-jugular fistula, Neck injury, Neurosurgery, Neurotherapeutics
INTRODUCTION
Approximately 25% of all penetrating neck traumas result in vascular injuries, and carotid artery injuries are particularly lethal.[
CASE REPORT
A 35-year-old male presented to us with a history of a stab injury to the right side of his neck with profuse bleeding 2 months before admission. Following the injury, he lost consciousness for 10 min and developed visual disturbance in the form of the left homonymous inferior quadrantanopia. He was neurologically intact and had GCS 15 at the time of presentation. An ill-defined cystic and compressible swelling just below the angle of the mandible was discovered on examination of the neck, which had a well-healed scar mark over it. The swelling had a palpable thrill and bruit that coincided with the apex beat. However, no abnormalities in cardiac status were discovered.
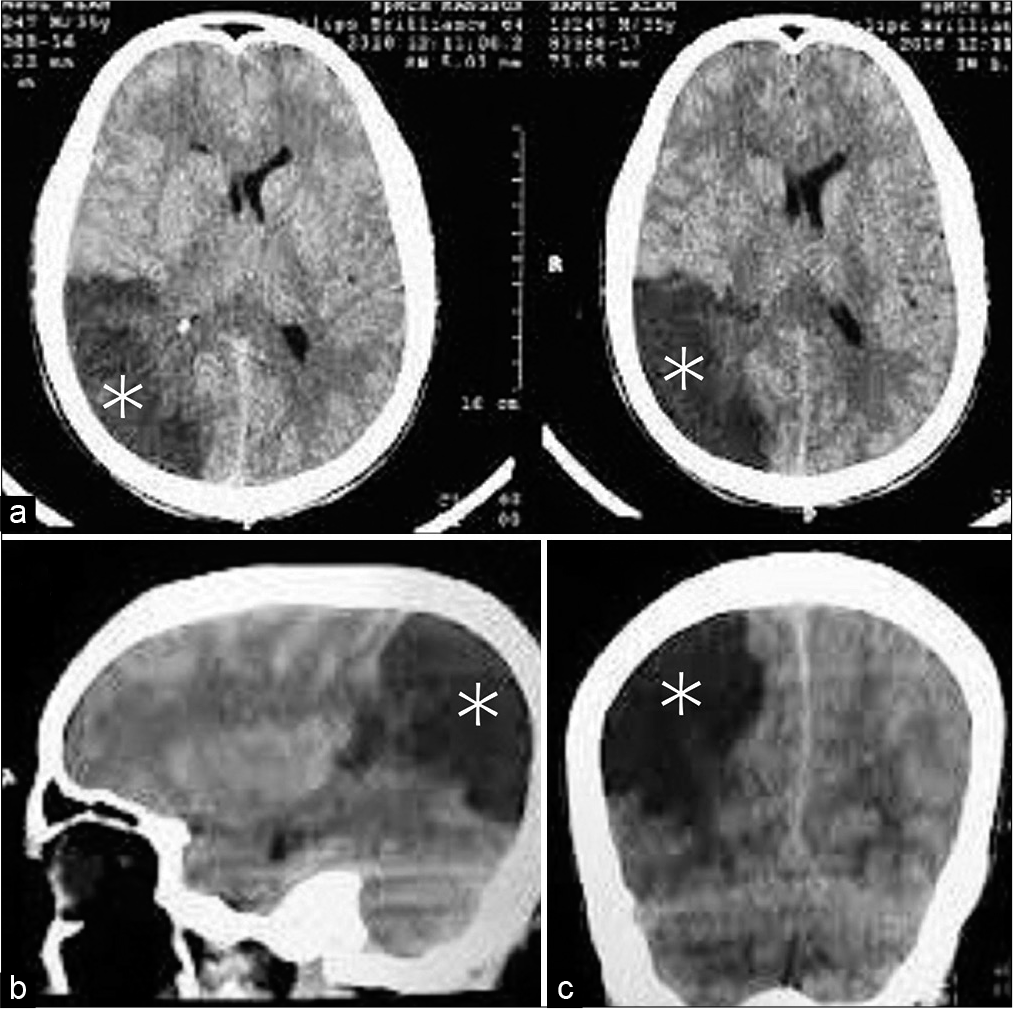
Although the patient had no significant neurological morbidity, a CT scan of the brain revealed a large right parieto-occipital infarction [
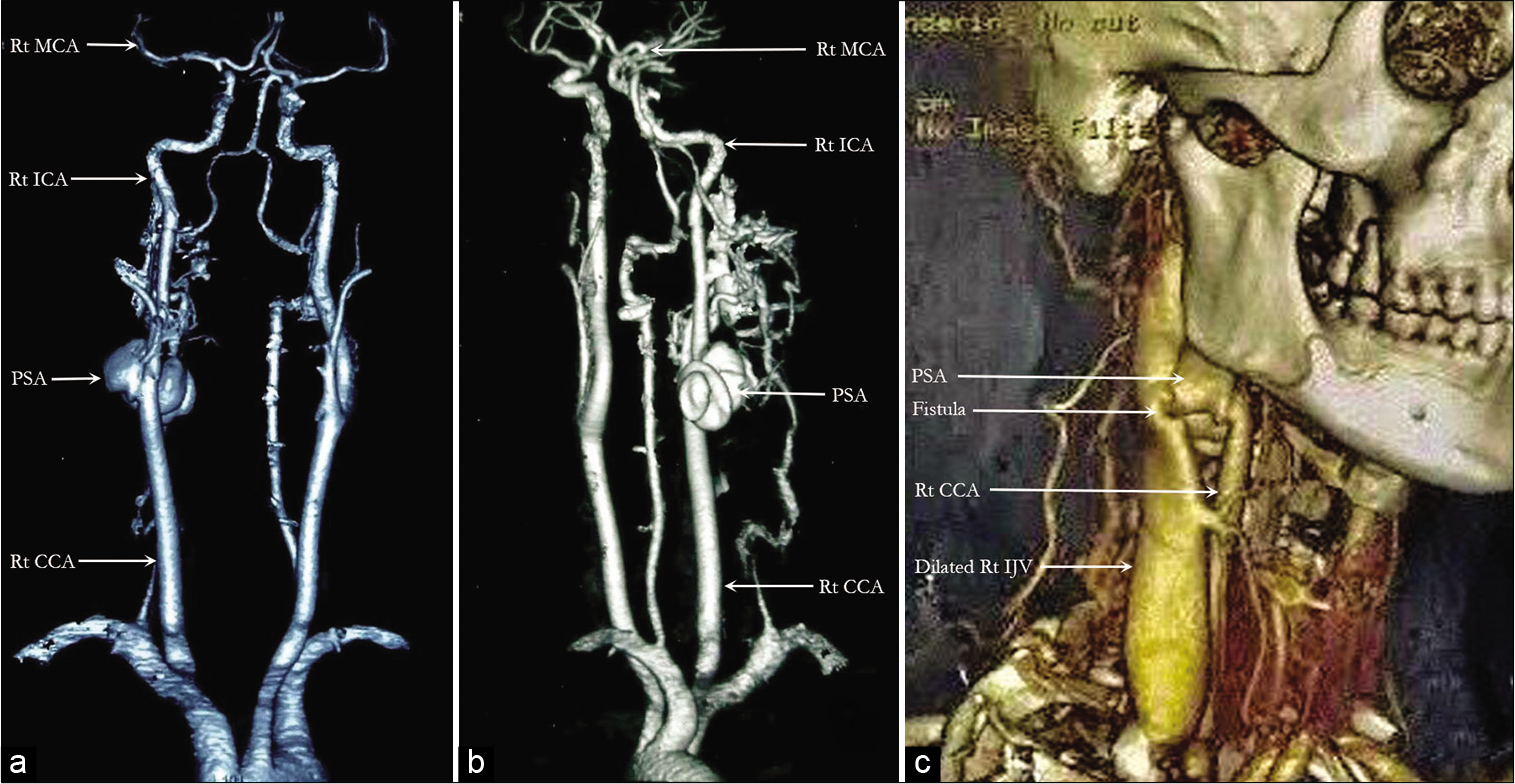
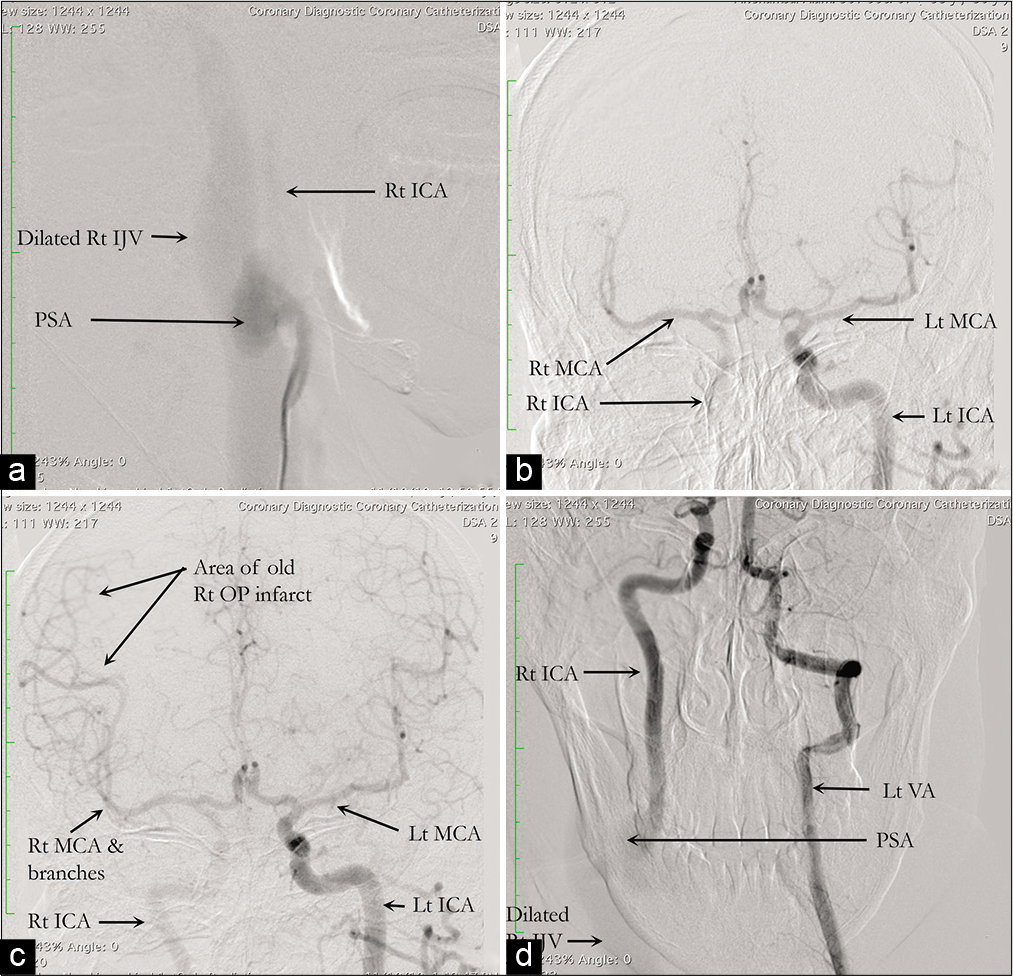
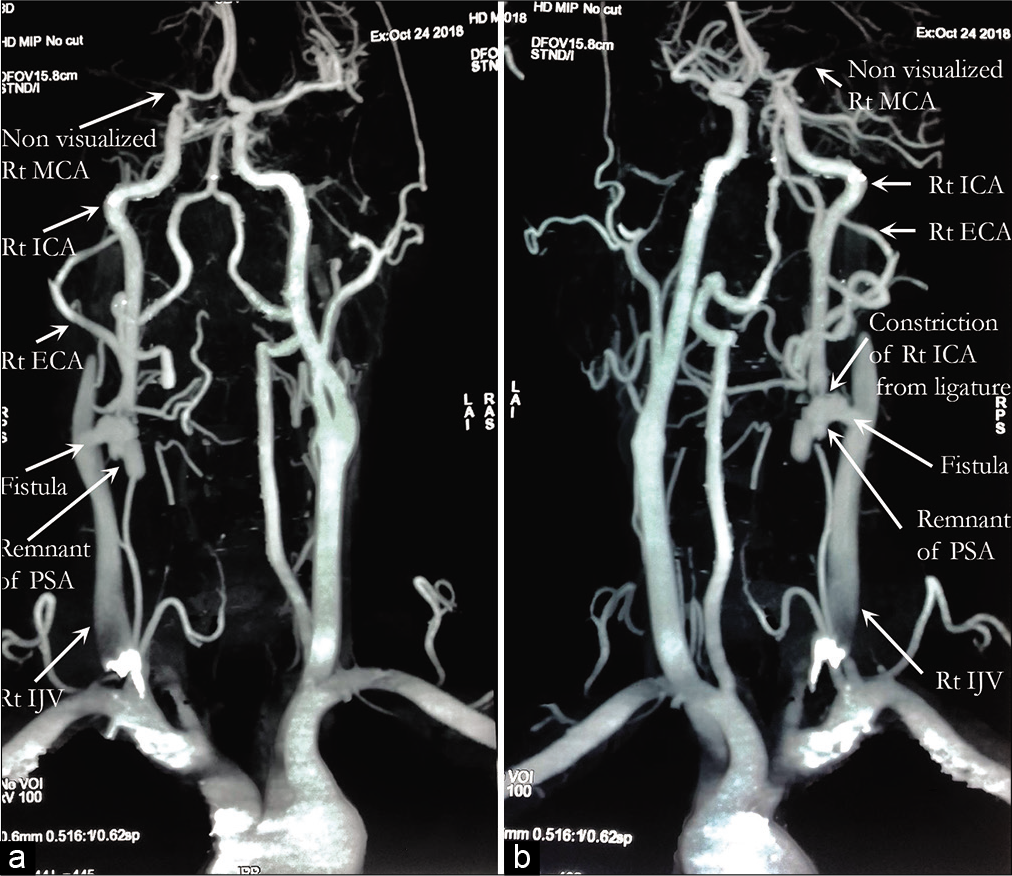
The surgery was planned to remove the PSA and repair the right ICA and IJV to reconnect the fistula. The PSA, measuring about 5 cm × 3 cm, was discovered just a short distance from the start of the ICA, and the fistula was discovered to be of such a large size that the surgical plan had to be revised intraoperatively. Bypass was also not an option, and because the left ICA fed the right cerebral hemisphere very well through the right MCA, the right ICA was ligated just above and below the connection with the PSA to trap it. Fistula ligation was also attempted, though due to its large caliber, only a ligature was attempted, and full obliteration was not possible. The PSA was discovered to originate at the junction of the CCA and ICA and was inseparable from the IJV, which was severely dilated.
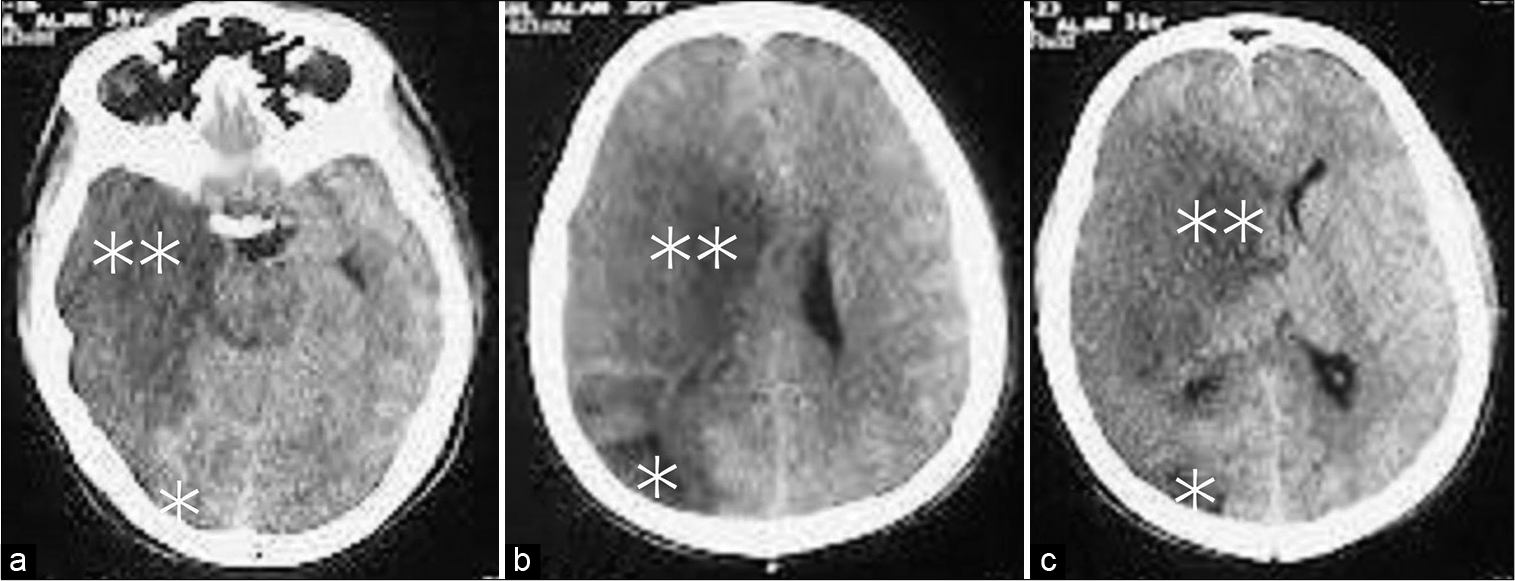
The patient was neurologically intact until the 2nd postoperative day (POD). On the 3rd POD, the patient suddenly deteriorated and became left hemiplegic with MRC Grade 0 in both the upper and lower limbs. GCS also dropped to 11, and the right pupil became dilated and nonresponsive to light. In addition to the previous right parieto-occipital infarct with significant midline shift, a CT scan of the brain revealed a large right-sided MCA territory infarct [
On the 2nd post discharge POD, GCS increased to 15, and the power of the left lower limb improved to MRC Grade 2 while the power of the left upper limb remained at MRC Grade 0. The right pupil was 6 mm in diameter and did not respond to light, whereas the left pupil was 4 mm in diameter and did respond to light. After 4 weeks of DC, cranioplasty was performed using autologous bone. After another 4 weeks of DC, the patient was discharged with MRC grades of 3 and 2, respectively, in the lower and upper limbs.
At 6 months, the patient’s left lower and upper limb power had improved to MRC Grades 4 and 3, respectively, and he could walk with assistance. However, the homonymous quadrantanopia remained unchanged.
DISCUSSION
AVF is an abnormal communication between an artery and a vein that occur as a result of penetrating trauma to both. An AVF is primarily a shunt that allows blood to flow from the arterial system with the higher pressure to the venous system with the lower pressure while bypassing high resistance arterioles.[
Penetrating injuries cause the majority of traumatic AVFs in the neck. Stab wounds, followed by gunshot wounds, are the most common modes of accidental injury that result in CJFs iatrogenic injury during the central venous catheter insertion of the IJV has recently been found to cause more CJFs than accidental injuries.[
Traumatic AVFs are frequently missed during the acute phase of injury, resulting in treatment being initiated weeks or even months after the initial trauma.[
To make a diagnosis of traumatic AVF and PSA, a careful history and physical examination are required. Nonetheless, additional noninvasive and invasive studies are required for definitive diagnosis and intervention planning, whether endovascular, conventional, or hybrid surgery are used.[
The investigations of choice are CTA, magnetic resonance angiography (MRA), Doppler ultrasonography, and DSA. All of these can identify the feeders and drainers, as well as the precise location and size of the fistula. Because of their noninvasiveness, CTA and MRA have largely replaced DSA in recent years. However, DSA has the added benefit of being able to perform interventional therapy at the same time.[
The neck is particularly vulnerable to external trauma, and its unparalleled intricate anatomy of close proximity of important neurovascular and aerodigestive structures makes the surgical approach to any pathology in the cervical region particularly difficult.[
Surgical intervention, whether endovascular, conventional, or hybrid, is the treatment of choice. The anatomy, size, and shape of the fistula and/or PSA, extent of the initial arterial injury, distal arterial flow, and facilities available should all be considered when deciding on a procedure.[
Repairing AVFs should be attempted as soon as possible if there is hemodynamic instability or evidence of vascular injury. Late surgery becomes difficult due to fibrosis and collateral development because the anatomy becomes distorted, and dissection becomes risky. The risk of hemorrhage may also increase with late surgery. Both AVFs and PSAs can be effectively treated with either traditional surgery or endovascular techniques.[
The primary goal of CJF management is to close the fistula and reconstruct the involved vessels. In general, open repair of a CJF by conventional surgical intervention includes end-to-end anastomoses, repair with patches, interposition grafts, or damage control procedures in some unavoidable situations. When reconstruction is impractical and the situation calls for it, ligation of the AVF’s contributing vessels should be considered. Endovascular techniques outperform surgical dissection, especially in cases of anatomic distortion, such as large AVFs associated with PSA, where they can be manipulated more easily than open surgery.[
Endovascular techniques for CJFs that are currently available include embolization with a balloon, coil, or glue, as well as the placement of covered stents or self-expandable graft stents. Advances in the development of biodegradable stents allow for the avoidance of stent-related complications. Endovascular therapy is a better choice of management because it has fewer complications than open surgery. Endovascular therapy also has less invasiveness, a lower risk of hemorrhage, and a shorter hospital stay. Furthermore, this allows for the selective occlusion of the fistula, which is strongly advised to avoid further complications.[
Because most CJFs can be effectively managed through open surgery, endovascular procedures, or a combination of the two, postsurgical complications have received little attention in the literature. The possibility of disaster from intraoperative blood loss, as well as injury to vital neurovascular and other surrounding structures, raises the risk of death from open surgery. Endovascular procedures, on the other hand, may increase the risk of thromboembolism, late intrastent stenosis, or even the formation of PSA at the site of arterial puncture.[
CONCLUSION
Penetrating neck injuries should be thoroughly evaluated for arteriovenous fistulae. To avoid complications, the common CJFs must be treated as soon as possible. Postoperative complications can be effectively managed with prompt action.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Commentary
The authors present an unusual case of traumatic carotidjugular fistula of high enough flow to cause a steal phenomenon. They should be commended for presenting their case as a cautionary example of a severe complication related to carotid sacrifice. It is always easy to be critical in retrospect, but previous work has shown that despite what would appear to be good collateral circulation on imaging studies, some patients will fail to tolerate carotid sacrifice, and the consequences can be significant. Safer options would have included an endovascular approach with ICA preservation, some form of cerebral revascularization to protect the hemisphere, trapping of the fistula with direct repair, or sacrifice of the internal jugular vein.
Eric Nussbaum, MD
Associate Editor-in-Chief, SNI: General Neurosurgery
National Brain Aneurysm & Tumor Center
Twin Cities, MN, USA
E-mail:
References
1. Asensio JA, Dabestani PJ, Miljkovic SS, Wenzl FA, Kessler JJ, Kalamchi LD. Traumatic penetrating arteriovenous fistulas: A collective review. Eur J Trauma Emerg Surg. 2021. p. 1-15
2. Bellosta R, Vescovi M, Attisani L, Luzzani L. Endovascular treatment of congenital external carotid-jugular fistula: Case report and review of the literature. Vasc Endovascular Surg. 2017. 51: 316-9
3. Caldarelli C, Biricotti M, Materazzi G, Spinelli C, Spisni R. Acquired carotid-jugular fistula: Its changing history and management. Int Sch Res Notices. 2013. 2013: 320241
4. Chew LS, Han JX, Ng YP, Ng HB. Surgical resection of carotidjugular arteriovenous fistula after multiple failed embolisation. BMJ Case Rep. 2019. 12: e228232
5. Cui D, Li J, Zeng G, Zhi X, Du J. A congenital external carotid artery-external jugular vein arteriovenous fistula was successfully treated by coil embolization (case report and literature review). Childs Nerv Syst. 2017. 33: 1583-7
6. de Oliveira Góes AM, Jeha SA, Dias DV, Ferreira JM. Surgical repair of a traumatic carotid-jugular arteriovenous fistula. J Vasc Bras. 2020. 19: e20200008
7. Elheis M, Aljarrah Q, Heis H, Haque A. Endovascular management of an acquired carotid-jugular fistula in a child. Am J Case Rep. 2018. 19: 839-49
8. Ezemba N, Ekpe EE, Ezike HA, Anyanwu CH. Traumatic common carotid-jugular fistula: Report of 2 cases. Tex Heart Inst J. 2006. 33: 81-3
9. Henry TC, Huei TJ, Yuzaidi M, Safri LS, Krishna K, Rizal IA. Unexpected complication of arteriovenous fistula of the left common carotid to internal jugular vein following central venous catheterization. Chin J Traumatol. 2020. 23: 29-31
10. Jenkins LN, Rezende-Neto JB. Current management of penetrating traumatic cervical vascular injuries. Curr Surg Rep. 2020. 8: 1-8
11. Jin SC, Lee DH, Huh CW. Self-expandable graft stenting in an iatrogenic fistula between common carotid artery and internal jugular vein. J Cerebrovasc Endovasc Neurosurg. 2017. 19: 213-6
12. Somerville C, Lucas LA, Biederman RW. Carotid-jugular fistula diagnosed by transesophageal echocardiography. Echocardiography. 2012. 29: E115-8
13. Wang C, Ge Y, Li L. Traumatic arteriovenous fistula of the left parotid region: A case report. Oncol Lett. 2016. 11: 3194-6
14. Warren JV, Nickerson JL, Elkin DC. The cardiac output in patients with arteriovenous fistulas. J Clin Invest. 1951. 30: 210-4