- Department of Neurology and Neurosurgery, IRCCS Galeazzi, Milan, Italy
Correspondence Address:
Domenico Servello
Department of Neurology and Neurosurgery, IRCCS Galeazzi, Milan, Italy
DOI:10.4103/2152-7806.187534
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Servello D, Zekaj E, Saleh C, Pacchetti C, Porta M. The pros and cons of intraoperative CT scan in evaluation of deep brain stimulation lead implantation: A retrospective study. Surg Neurol Int 02-Aug-2016;7:
How to cite this URL: Servello D, Zekaj E, Saleh C, Pacchetti C, Porta M. The pros and cons of intraoperative CT scan in evaluation of deep brain stimulation lead implantation: A retrospective study. Surg Neurol Int 02-Aug-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/pros-cons-intraoperative-ct-scan-evaluation-deep-brain-stimulation-lead-implantation-retrospective-study/
Abstract
Background:Deep brain stimulation (DBS) is an established therapy for movement disorders, such as Parkinson's disease (PD), dystonia, and tremor. The efficacy of DBS depends on the correct lead positioning. The commonly adopted postoperative radiological evaluation is performed with computed tomography (CT) scan and/or magnetic resonance imaging (MRI).
Methods:We conducted a retrospective study on 202 patients who underwent DBS from January 2009 to October 2013. DBS indications were PD, progressive supranuclear palsy, tremor, dystonia, Tourette syndrome, obsessive compulsive disorder, depression, and Huntington's disease. Preoperatively, all patients underwent brain MRI and brain CT scan with the stereotactic frame positioned. The lead location was confirmed intraoperatively with CT. The CT images were subsequently transferred to the Stealth Station Medtronic and merged with the preoperative planning. On the first or second day after, implantation we performed a brain MRI to confirm the correct position of the lead.
Results:In 14 patients, leads were in suboptimal position after intraoperative CT scan positioning. The cases with alteration in the Z-axis were corrected immediately under fluoroscopic guidance. In all the 14 patients, an immediate repositioning was done.
Conclusions:Based on our data, intraoperative CT scan is fast, safe, and a useful tool in the evaluation of the position of the implanted lead. It also reduces the patient's discomfort derived from the transfer of the patient from the operating room to the radiological department. However, intraoperative CT should not be considered as a substitute for postoperative MRI.
Keywords: Accuracy, computed tomography, deep brain stimulation, imaging, magnetic resonance imaging, O-Arm, targets
INTRODUCTION
Since the renaissance of deep brain stimulation (DBS) in 1987 approximately 100,000 patients have been implanted. Initially, DBS was applied for movement disorders, such as Parkinson's disease (PD),[
The clinical efficacy of DBS is multifactorial, and correct patient selection is fundamental. Tailored target selection on patient symptomatology is equally of critical importance. Finally, a perfect lead positioning is required in order to obtain the best clinical results. DBS is a highly precise surgical procedure, wherein a millimetric error might lead to poor clinical results or to adverse stimulation effects. In most centers, intraoperative final lead position is valuated with lateral and anterior–posterior X-rays, whereas outside the operating room a ventriculography, stereotactic computed tomography (CT) scan or magnetic resonance imaging (MRI) are imaging options.[
In this paper, we present our large experience with intraoperative CT imaging (O-Arm Medtronic, Medtronic, Minneapolis, Minnesota, 55432-5604, USA) in DBS lead evaluation. Our objective is to discuss the safety, effectiveness, advantages, and disadvantages of this procedure.
MATERIALS AND METHODS
We have 202 patients who underwent DBS at our Institute from June 2009 to December 2014 for PD, dystonia, essential tremor, progressive supranuclear palsy (PSP), TS, OCD, and depression. The selected targets were the subthalamic nucleus (STN) for PD, the posterior-ventral globus pallidus internus (GPi) for PD, the ventral-intermedia nucleus (VIM) for essential tremor, the pedunculopontine nucleus (PPN) for PSP, the nucleus-accumbens-anterior limb of internal capsule (NA-ALIC) for TS, and the ventroral-centromedian-parafascicular complex (VO-CM/Pf) and the anterior-medial GPi for TS.
Preoperatively, one to two days prior to the surgery all patients underwent brain MRI (1.5 T Avanto Siemens, Avanto Siemens Medical Solutions USA, Inc, Malvem, PA 19355, USA), and following sequences were used: In all patients volumetric gadolinium enhanced T1-weighted sequences of 1 mm of slice thickness were obtained; for STN, NA-ALIC, GPi posterior-ventral (p.v) and PPN targeting a 2 mm T2-weighted axial and coronal sequences were also added; and for Gpi p.v and PPN axial 2 mm DPI sequences were added. On the day of surgery, we positioned the head frame Cosmann–Roberts–Wells under local anesthesia and performed a stereotactic brain CT scan. All images subsequently were sent to a Neuro-Navigation Stealth Station System (Treon Medtronic, Medtronic Navigation Louisville, CO 80027 USA), where MRI images were fused to the stereotactic CT images. Subsequently, the Talairach Grid was uploaded to reformat the MRI images in order to produce images orthogonal to the anterior commissure-posterior commissure (AC-PC) line and sagittal plane. In PD or dystonia patients, the Schaltenbrand–Wahren atlas was also applied. In cases, in which an optimal correspondence between the atlas and the patient's MRI was observed, the target coordinates given by the atlas were chosen. Whereas in other patients with poor correspondence between the target and the atlas, the target was directly visualized on MRI images; for STN targeting, the area chosen was at the dorsal-lateral part of the STN identified as the biconvex hypointense area in the axial T2 sequences, the target area lies lateral to the red nucleus at the level of its anterior border; for the posterior-ventral GPi the area identified was at the posterior part of the GPi at the cross point with the optic tract that lies immediately below the target. The NA-ALIC was directly visualized on axial and coronal T2 sequences. In TS patients, the VO-CM/Pf was targeted at the fixed coordinates of 5 mm lateral, 2 mm posterior to mid-commissural point at the level of the AC-PC plane. After determination of the target coordinates, the patient was transferred to the operating room and the surgery was started. Procedures were performed under local anesthesia, and mild sedation (dexmedetomidine) was given in some particular cases. In some TS patients, due to relevant tics the procedure occurred under general anesthesia.
Patient positioning
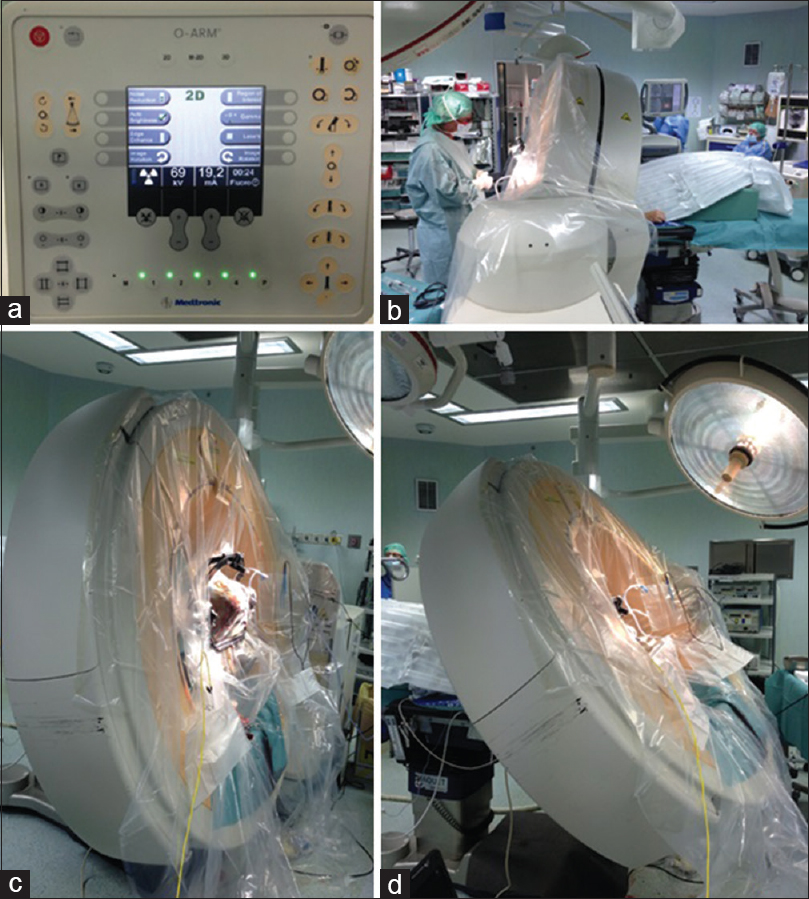
Patient positioning is complex when using O-ARM. The operating table should be shifted toward the head position in order to be as far as possible from the base to give free access to the O-ARM. Patient's head frame Mayfield part, fixed to the operating table, should be in the vertical position in order to allow a free mobility of the acquiring part of the O-Arm. The intraoperative CT scan is positioned on the head of the operating table and different positions of the acquiring part must be memorized in order to allow safe movement of the device, avoiding collision with the frame or with the operating table [
Figure 1
(a) Several positions are memorized, which allows the safe movement of the device (b) the device positioned during surgery, on the left the surgical part and on the right part the anesthetist and neurologist area; (c) the device positioned in the working position is seen; it is imperative that there is no obstacle for the surgeon; (d) the device in the acquiring position
Surgical procedure
We used 1 to 3 microelectrodes that advanced 0.5 mm every 30 s starting 10 mm above the target up to 1–3 mm below the target. After microrecording (MER), we performed macrostimulation to evaluate for adverse events or clinical improvement. After choosing the best site to stimulate, we performed anterior–posterior and lateral X-rays with the guides in the position. We then positioned the lead and repeated the anterior–posterior and lateral X-ray to assess for any unwanted displacement of the lead. After positioning and fixing both electrodes with Stim-lock, we performed intraoperative CT imaging, with images sent to the Neuronavigation Stealth Station. Intraoperative O-Arm images were fused with the preoperative brain stereotactic CT scan and the brain MRI. The final lead position was compared with the preoperative trajectories and the selected target coordinates. The lead was repositioned if it had a distance from the chosen target of more than 2 mm. On the first or second day after electrode implantation and before internalization and IPG positioning, all patients underwent brain MRI to confirm the correct lead position.
In the first 20 patients, we performed postsurgical stereotactic CT scan images and an intraoperative CT scan with O-Arm, and both images were sent to the Neuronavigation Stealth Station and the coordinates were compared.
RESULTS
In the first 20 patients, the coordinates obtained with the intraoperative O-Arm and post surgical stereotactic CT-scan were identical. Because of this confirmation of the reliability of intraoperative O-Arm images, in all subsequent cases we used only intraoperative O-Arm images to confirm lead positioning.
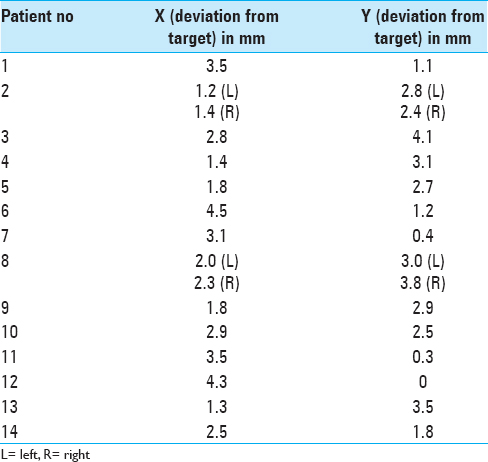
Of the 202 evaluated patients, in 14 patients (6.9%) (16 electrodes, in two patients both sides), leads were in the suboptimal position (distance greater than 2 mm from the chosen target) after intraoperative CT scans [
DISCUSSION
DBS is a validated procedure for movement disorders such as PD, dystonia, and tremor and is emerging as a promising therapeutic option for psychiatric diseases.[
Efficacy in evaluating lead position
One of the main concerns of functional surgeons is the correct lead position. In a 2006 review, lead misplacement occurred in approximately 5% of cases,[
A detailed description of the procedure with intraoperative CT scan with O-Arm has been that of the group lead by Starr.[
In our experience, we did not find any difference between intraoperative CT scan electrode coordinates obtained with O-Arm and electrode coordinates obtained with postsurgical stereotactic CT scan. In our series, we had to reposition leads in 14 out of 202 patients after detection of the error in lead positioning by intraoperative O-Arm CT scan. A need to reposition the electrode was considered as misplacement that exceeded 2 mm from the chosen area. The lead misplacement in 6 cases was secondary to dura mater lead displacement, which required a further opening and coagulation of the dura mater prior to lead repositioning; in 3 cases, lead was misplaced probably during the fixing of the lead to the skull because in these cases it has been particularly complex fixing to the skull, whereas in the remaining three cases we did not find any reasonable cause of lead misplacement. In 9 cases, the cause of the error was technical, during lead fixation to the skull, or after implantation due to dura mater. Considering that during surgery MER and macro-stimulation lead position was optimal, here the leads were directly positioned in the chosen stereotactic coordinates without having to repeat all surgical steps. In the 3 patients, where we did not find any reason for the error, the entire procedure with MER and macro-stimulation was repeated.
In 2 patients, where the intraoperative CT scan did not show an error, the patients had relevant pneumoencephalus. We think that this condition determined a considerable brain shift and hence the correct stereotactic coordinates did not match with the correct anatomical site. A stereotactic CT scan could miss such an error whereas a more anatomic imaging such as MRI is reliable for these cases. We repeated surgery after complete air absorption.
Safety
A safe procedure is defined as a procedure that does not cause immediate or tardive side effects. Immediate side effects with intraoperative imaging techniques may result from a run-in between the device and the patient, frame, or other parts in the operating room. We had no adverse events during all imaging procedures, i.e., we had no patient injury during movement of the device for the acquisition of the images. We have to underline that, in the first patients, we had some difficulties in finding the most optimal working position for the intraoperative CT, which would be comfortable for the surgeon, for the patient, and the anesthetist. With experience, we solved this issue by correcting the position of the parts of the head frame and the position of the device with the operating table. Equally crucial is that during the movement of the device, the sterility is always maintained, which we solved with a large transparent drape.
With respect to the tardive side effects, one of the main concerns are effects of radiation. Zhang et al. demonstrated that the O-arm CT scanner delivers approximately 50% of the radiation dose of a routine 64-slice CT scanner.[
Advantages
First of all an intraoperative evaluation reduces the discomfort of the patient, the surgeon, and the anesthetist caused by the patient's transfer from the operating room to the radiological unit. It reduces the time of control or the time of repositioning in cases of lead misplacement. Furthermore, in patients undergoing DBS under general anesthesia, the transfer is more difficult and after imaging the patient has to return to the operating room to bring the patient out of anesthesia. One should pay attention to those cases where correct implantation is difficult. In 3 patients, without knowing the exact cause of lead misplacement, the lead was in a suboptimal position after more than one attempt. Not using the O-arm in these cases results in having to transfer the patient to the radiology department and then back to the operating room. Immediate intraoperative imaging evaluation reduces the time of the procedure.
Disadvantages
Our intraoperative CT scan does not have the software for elaboration of the images of the brain parenchyma so it visualizes only the electrodes. Early hemorrhages are, therefore, not detected by this exam.
Another potential limitation is the airway control by the anesthetist. In local anesthesia procedure, anesthetist should have the possibility of prompt and easy airway control. We had 8 patients who had an epileptic crisis that required an interruption of the procedure and breathing assistance after lorazepam administration. In all cases, a rapid removal of the sterile part, head frame, and of the O-Arm was done with prompt airway control by the anesthetist. Anesthetists did not find any differences in emergence management with the previous cases of epileptic crisis during DBS performed with C-ARM.
Surgeon comfort during surgical procedure is another critical point. In the first procedures, the surgeon was not comfortable during surgery due to the cumbersome O-Arm in comparison to the X-ray device. However, with time, we optimized the position of the O-Arm in order to have the patient head as out as possible, and to date there is no difference with the previous X-ray's position.
CONCLUSIONS
Intraoperative CT scan is safe, efficacious, and a fast procedure that helps reducing the time of evaluation of DBS lead position.[
Particular attention must be reserved for patients with relevant pneumoencephalus as leads might be positioned in the correct stereotactic area but due to brain shift maybe localized in a wrong anatomical area. Further, it is important to consider that this examination misses the intraparenchymal hemorrhages, so in all patients in whom there is a suspicion of intracranial hemorrhage a brain CT scan should be performed. Furthermore, this exam, despite the abovementioned disadvantages, exposes the patient to a lower dose of radiation than conventional CT scan, thus along with comparable evaluation has the advantages of lower radiation dose.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Benabid AL, Pollak P, Gervason C, Hoffmann D, Gao DM, Hommel M. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet. 1991. 337: 403-6
2. Benabid AL, Pollak P, Gross C, Hoffmann D, Benazzouz A, Gao DM. Acute and long-term effects of subthalamic nucleus stimulation in Parkinson's disease. Stereotactic Functional Neurosurg. 1994. 62: 76-84
3. Fiegele T, Feuchtner G, Sohm F, Bauer R, Anton JV, Gotwald T. Accuracy of stereotactic electrode placement in deep brain stimulation by intraoperative computed tomography. Parkinsonism Relat Disord. 2008. 14: 595-9
4. Hamani C, Lozano AM. Hardware-related complications of deep brain stimulation: A review of the published literature. Stereotactic Functional Neurosur. 2006. 84: 248-51
5. Holloway K, Docef A. A quantitative assessment of the accuracy and reliability of O-arm images for deep brain stimulation surgery. Neurosurg. 2013. 72: S47-57
6. Kupsch A, Kuehn A, Klaffke S, Meissner W, Harnack D, Winter C. Deep brain stimulation in dystonia. J Neurol. 2003. 250: SI47-52
7. Laxton AW, Lozano AM. Deep brain stimulation for the treatment of Alzheimer disease and dementias. World Neurosurg. 2013. 80: S28 e1-8
8. Lee JY, Jeon BS, Paek SH, Lim YH, Kim MR, Kim C. Reprogramming guided by the fused images of MRI and CT in subthalamic nucleus stimulation in Parkinson disease. Clin Neurol Neurosurg. 2010. 112: 47-53
9. Lee JY, Kim JW, Lee JY, Lim YH, Kim C, Kim DG. Is MRI a reliable tool to locate the electrode after deep brain stimulation surgery. Comparison study of CT and MRI for the localization of electrodes after DBS?. Acta Neurochir. 2010. 152: 2029-36
10. Lee MW, De Salles AA, Frighetto L, Torres R, Behnke E, Bronstein JM. Deep brain stimulation in intraoperative MRI environment - comparison of imaging techniques and electrode fixation methods. Minim Invasive Neurosurg. 2005. 48: 1-6
11. Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C. Deep brain stimulation for treatment-resistant depression. Neuron. 2005. 45: 651-60
12. Nuttin B, Cosyns P, Demeulemeester H, Gybels J, Meyerson B. Electrical stimulation in anterior limbs of internal capsules in patients with obsessive-compulsive disorder. Lancet. 1999. 354: 1526-
13. Okun MS, Tagliati M, Pourfar M, Fernandez HH, Rodriguez RL, Alterman RL. Management of referred deep brain stimulation failures: A retrospective analysis from 2 movement disorders centers. Arch Neurol. 2005. 62: 1250-5
14. Paek SH, Han JH, Lee JY, Kim C, Jeon BS, Kim DG. Electrode position determined by fused images of preoperative and postoperative magnetic resonance imaging and surgical outcome after subthalamic nucleus deep brain stimulation. Neurosurg. 2008. 63: 925-36
15. Pinto S, Le Bas JF, Castana L, Krack P, Pollak P, Benabid AL. Comparison of two techniques to postoperatively localize the electrode contacts used for subthalamic nucleus stimulation. Neurosurg. 2007. 60: 285-92
16. Saleh C, Fontaine D. Deep brain stimulation for psychiatric diseases: What are the risks?. Curr Psychiatry Rep. 2015. 17: 33-
17. Shahlaie K, Larson PS, Starr PA. Intraoperative computed tomography for deep brain stimulation surgery: Technique and accuracy assessment. Neurosurg. 2011. 68: 114-24
18. Sharma M, Deogaonkar M. Accuracy and safety of targeting using intraoperative “O-arm” during placement of deep brain stimulation electrodes without electrophysiological recordings. J Clin Neurosci. 2016. 15: S0967-5868
19. Shin M, Penholate MF, Lefaucheur JP, Gurruchaga JM, Brugieres P, Nguyen JP. Assessing accuracy of the magnetic resonance imaging-computed tomography fusion images to evaluate the electrode positions in subthalamic nucleus after deep-brain stimulation. Neurosurg. 2010. 66: 1193-202
20. Starr PA, Martin AJ, Larson PS. Implantation of deep brain stimulator electrodes using interventional MRI. Neurosurg Clin N Am. 2009. 20: 193-203
21. Starr PA, Martin AJ, Ostrem JL, Talke P, Levesque N, Larson PS. Subthalamic nucleus deep brain stimulator placement using high-field interventional magnetic resonance imaging and a skull-mounted aiming device: Technique and application accuracy. J Neurosurg. 2010. 112: 479-90
22. Vandewalle V, van der Linden C, Groenewegen HJ, Caemaert J. Stereotactic treatment of Gilles de la Tourette syndrome by high frequency stimulation of thalamus. Lancet. 1999. 353: 724-
23. Wu H, Van Dyck-Lippens PJ, Santegoeds R, van Kuyck K, Gabriels L, Lin G. Deep-brain stimulation for anorexia nervosa. World Neurosurg. 2013. 80: S29-e1-10
24. Zhang K, Bhatia S, Oh MY, Cohen D, Angle C, Whiting D. Long-term results of thalamic deep brain stimulation for essential tremor. J Neurosurg. 2010. 112: 1271-6