- Cerebrovascular Research Laboratory, Department of Neurosurgery, Emory University School of Medicine, Atlanta, Georgia, USA
- Mercer University School of Medicine, Savannah, Georgia, USA
Correspondence Address:
Gustavo Pradilla
Cerebrovascular Research Laboratory, Department of Neurosurgery, Emory University School of Medicine, Atlanta, Georgia, USA
DOI:10.4103/sni.sni_88_18
Copyright: © 2018 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Jack W. Barrow, Nefize Turan, Pasang Wangmo, Anil K. Roy, Gustavo Pradilla. The role of inflammation and potential use of sex steroids in intracranial aneurysms and subarachnoid hemorrhage. 26-Jul-2018;9:150
How to cite this URL: Jack W. Barrow, Nefize Turan, Pasang Wangmo, Anil K. Roy, Gustavo Pradilla. The role of inflammation and potential use of sex steroids in intracranial aneurysms and subarachnoid hemorrhage. 26-Jul-2018;9:150. Available from: http://surgicalneurologyint.com/surgicalint-articles/the-role-of-inflammation-and-potential-use-of-sex-steroids-in-intracranial-aneurysms-and-subarachnoid-hemorrhage/
Abstract
Background:Aneurysmal subarachnoid hemorrhage (aSAH) continues to be a devastating neurological condition with a high risk of associated morbidity and mortality. Inflammation has been shown to increase the risk of complications associated with aSAH such as vasospasm and brain injury in animal models and humans. The goal of this review is to discuss the inflammatory mechanisms of aneurysm formation, rupture and vasospasm and explore the role of sex hormones in the inflammatory response to aSAH.
Methods:A literature review was performed using PubMed using the following search terms: “intracranial aneurysm,” “cerebral aneurysm,” “dihydroepiandrosterone sulfate” “estrogen,” “hormone replacement therapy,” “inflammation,” “oral contraceptive,” “progesterone,” “sex steroids,” “sex hormones” “subarachnoid hemorrhage,” “testosterone.” Only studies published in English language were included in the review.
Results:Studies have shown that administration of sex hormones such as progesterone and estrogen at early stages in the inflammatory cascade can lower the risk and magnitude of subsequent complications. The exact mechanism by which these hormones act on the brain, as well as their role in the inflammatory cascade is not fully understood. Moreover, conflicting results have been published on the effect of hormone replacement therapy in humans. This review will scrutinize the variations in these studies to provide a more detailed understanding of sex hormones as potential therapeutic agents for intracranial aneurysms and aSAH.
Conclusion:Inflammation may play a role in the pathogenesis of intracranial aneurysm formation and subarachnoid hemorrhage, and administration of sex hormones as anti-inflammatory agents has been associated with improved functional outcome in experimental models. Further studies are needed to determine the therapeutic role of these hormones in the intracranial aneurysms and aSAH.
Keywords: Estrogen, inflammation, intracranial aneurysms, progesterone, sex hormones, subarachnoid hemorrhage
INTRODUCTION
Patients surviving an aneurysmal subarachnoid hemorrhage (aSAH) often develop cerebral vasospasm and delayed ischemic neurological injury.[
Role of inflammation in intracranial aneurysms and subarachnoid hemorrhage
Evidence of inflammation in aneurysm formation and rupture
Factors leading to abnormal vascular remodeling and weakening of the vessel wall are not well understood, but chronic inflammation and infiltration of inflammatory cells has been shown to be an early histologic hallmark for aneurysms.[
Evidence of inflammatory markers in the systemic circulation and cerebrospinal fluid after subarachnoid hemorrhage
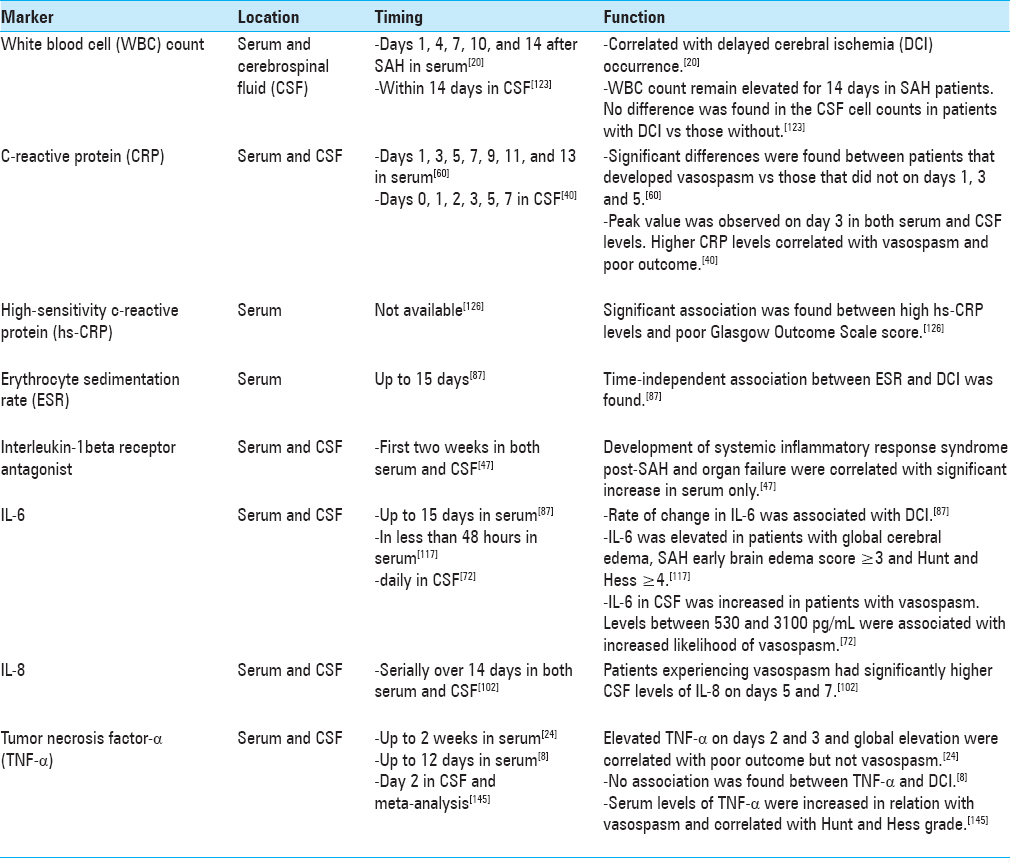
Inflammatory markers increase in the systemic circulation as well as in cerebrospinal fluid (CSF) following SAH and are predictive of poor outcomes.[
Several studies have investigated various inflammatory mediators in cerebrospinal fluid (CSF) following aSAH, with some conflicting reports.[
Evidence for inflammation as a cause of vasospasm after subarachnoid hemorrhage
Several clinical studies have attempted to correlate fever and inflammation in the absence of infection with vasospasm.[
Recent studies have been done to explore a possible genetic predisposition to vasospasm. One promising avenue has been the study of haptoglobin proteins, which are responsible for removal of free hemoglobin from CSF that may be the cause of inflammation. Haptoglobin (Hp) has three known distinct phenotypes in humans – Hp1-1, Hp2-1, and Hp2-2.[
Changes in NO have also been extensively studied in the induction of vasospasm. Increase in the levels of endothelial nitric oxide synthase (eNOS) and inducible nitric oxide synthase[
ET-1, a potent vasoconstrictor, is thought to play a role in the inflammatory response after SAH.[
Several studies have shown a relationship between glutamate, as well as a synthetic analog N-methyl-D-aspartate (NMDA), and vasodilation under physiological conditions.[
The impact of sex hormones on intracranial aneurysms and subarachnoid hemorrhage
Estrogen
Estrogen is the primary female sex hormone responsible for the development and regulation of the female reproductive system, although it has been found to play a role in male physiology as well.[
Estrogen is thought to play a role in aneurysm formation. Females have been shown to develop intracranial aneurysms at higher rates than males and, experimental animal studies support the hypothesis that induced estrogen deficiency via bilateral oophorectomy in rats causes an increase in the frequency of aneurysm formation and augment the aneurysm size.[
Evidence obtained from animal studies suggests that continuous estrogen treatment in SAH-induced rats may decrease the rate and severity of vasospasm by inhibiting endothelin-1 production, increasing iNOS expression, and preserving eNOS expression.[
Progesterone
Progesterone (PROG) is another sex steroid naturally synthesized by neurons and oligodendrocytes in the CNS. In addition to its hypothalamic receptors involved in the regulation of female reproductive physiology, PROG receptors are constitutively expressed in other parts of the brain including the cerebral cortex, hippocampus, basal ganglia, and cerebellum.[
Progesterone may play a critical role in altering the pathogenesis of SAH, and has already been proven to be beneficial in few studies of experimental SAH.[
Testosterone and dihydroepiandosterone sulfate
Testosterone, another gonadal sex steroid, also plays important roles in the CNS, but its direct role in SAH is still unclear.[
Dihydroepiandrosterone sulfate (DHEAS) is another sex steroid recently associated with favorable outcomes in human SAH.[
Oral contraceptives and hormone replacement therapy
Several population-based studies have failed to show a strong association between risk of SAH and the use of oral contraceptives.[
Translation from bench to bedside and remaining challenges with clinical trials
Though there is promising data alluding to sex hormones as potential therapeutic agents for vasospasm and neuroprotection in aSAH patients, the gap between animal studies and human trials is still large. Concern surrounding the failure of clinical trials evaluating progesterone in TBI in humans despite extensive supporting data in animal models calls for more precise outcome measures and alternative clinical trial methodologies.[
CONCLUSION
Inflammation in the CNS is a major contributing force behind vasospasm and early brain injury in aSAH patients. Though this link has been made in many animal experiments, human trials with anti-inflammatory agents have not been successful in reducing morbidity and mortality and improving functional outcome. Evaluation of sex hormones as potential therapeutic agents to stabilize intracranial aneurysms and improve functional outcome in aSAH patients is promising as many preliminary animal studies indicate the safety and effectiveness of the sex steroids to cross the BBB. Future studies are warranted to determine the role of sex hormones in treatment of these conditions.
Financial support and sponsorship
Nil.
Disclosure/Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
1. Aihara Y, Kasuya H, Onda H, Hori T, Takeda J. Quantitative analysis of gene expressions related to inflammation in canine spastic artery after subarachnoid hemorrhage. Stroke. 2001. 32: 212-7
2. Aoki T, Kataoka H, Ishibashi R, Nozaki K, Egashira K, Hashimoto N. Impact of monocyte chemoattractant protein-1 deficiency on cerebral aneurysm formation. Stroke. 2009. 40: 942-51
3. Aoki T, Kataoka H, Morimoto M, Nozaki K, Hashimoto N. Macrophage-derived matrix metalloproteinase-2 and -9 promote the progression of cerebral aneurysms in rats. Stroke. 2007. 38: 162-9
4. Aoki T, Kataoka H, Shimamura M, Nakagami H, Wakayama K, Moriwaki T. NF-kappaB is a key mediator of cerebral aneurysm formation. Circulation. 2007. 116: 2830-40
5. Arthur JS, Ley SC. Mitogen-activated protein kinases in innate immunity. Nature reviews. Immunology. 2013. 13: 679-92
6. Ayala C, Croft JB, Greenlund KJ, Keenan NL, Donehoo RS, Malarcher AM. Sex differences in US mortality rates for stroke and stroke subtypes by race/ethnicity and age, 1995-1998. Stroke. 2002. 33: 1197-201
7. Ayres S, Abplanalp W, Liu JH, Subbiah MT. Mechanisms involved in the protective effect of estradiol-17beta on lipid peroxidation and DNA damage. Am J Physiol. 1998. 274: E1002-8
8. Beeftink MM, Ruigrok YM, Rinkel GJ, van den Bergh WM. Relation of serum TNF-alpha and TNF-alpha genotype with delayed cerebral ischemia and outcome in subarachnoid hemorrhage. Neurocrit Care. 2011. 15: 405-9
9. Bell JD, Thomas TC, Lass E, Ai J, Wan H, Lifshitz J. Platelet-mediated changes to neuronal glutamate receptor expression at sites of microthrombosis following experimental subarachnoid hemorrhage. J Neurosurg. 2014. 121: 1424-31
10. Belle MD, Lea RW. Androgen receptor immunolocalization in brains of courting and brooding male and female ring doves (Streptopelia risoria). Gen Comp Endocrinol. 2001. 124: 173-87
11. Białek M, Zaremba P, Borowicz KK, Czuczwar SJ. Neuroprotective role of testosterone in the nervous system. Pol J Pharmacol. 2004. 56: 509-18
12. Bonita R. Cigarette smoking, hypertension and the risk of subarachnoid hemorrhage: A population-based case-control study. Stroke. 1986. 17: 831-5
13. Borsody M, Burke A, Coplin W, Miller-Lotan R, Levy A. Haptoglobin and the development of cerebral artery vasospasm after subarachnoid hemorrhage. Neurology. 2006. 66: 634-40
14. Bowman BH, Kurosky A. Haptoglobin: The evolutionary product of duplication, unequal crossing over, and point mutation. Adv Hum Genet. 1982. 12: 189-261
15. Bowman G, Dixit S, Bonneau RH, Chinchilli VM, Cockroft KM. Neutralizing antibody against interleukin-6 attenuates posthemorrhagic vasospasm in the rat femoral artery model. Neurosurgery. 2004. 54: 719-25
16. Bruce-Keller AJ, Keeling JL, Keller JN, Huang FF, Camondola S, Mattson MP. Antiinflammatory effects of estrogen on microglial activation. Endocrinology. 2000. 141: 3646-56
17. Busija DW, Bari F, Domoki F, Louis T. Mechanisms involved in the cerebrovascular dilator effects of N-methyl-d-aspartate in cerebral cortex. Brain Res Rev. 2007. 56: 89-100
18. Chaichana KL, Levy AP, Miller-Lotan R, Shakur S, Tamargo RJ. Haptoglobin 2-2 genotype determines chronic vasospasm after experimental subarachnoid hemorrhage. Stroke. 2007. 38: 3266-71
19. Chaichana KL, Pradilla G, Huang J, Tamargo RJ. Role of inflammation (leukocyte-endothelial cell interactions) in vasospasm after subarachnoid hemorrhage. World Neurosurg. 2010. 73: 22-41
20. Chamling B, Gross S, Stoffel-Wagner B, Schubert GA, Clusmann H, Coburn M. Early Diagnosis of Delayed Cerebral Ischemia: Possible Relevance for Inflammatory Biomarkers in Routine Clinical Practice?. World Neurosurg. 2017. 104: 152-7
21. Chang CM, Su YF, Chang CZ, Chung CL, Tsai YJ, Loh JK. Progesterone attenuates experimental subarachnoid hemorrhage-induced vasospasm by upregulation of endothelial nitric oxide synthase via Akt signaling pathway. Biomed Res Int 2014. 2014. 207616:
22. Chen G, Tariq A, Ai J, Sabri M, Jeon HJ, Tang EJ. Different effects of clazosentan on consequences of subarachnoid hemorrhage in rats. Brain Res. 2011. 1392: 132-9
23. Chen YH, Cheng ZY, Shao LH, Shentu HS, Fu B. Macrophage migration inhibitory factor as a serum prognostic marker in patients with aneurysmal subarachnoid hemorrhage. Clin Chim Acta. 2017. 473: 60-4
24. Chou SH, Feske SK, Atherton J, Konigsberg RG, De Jager PL, Du R. Early elevation of serum tumor necrosis factor-alpha is associated with poor outcome in subarachnoid hemorrhage. J Investig Med. 2012. 60: 1054-8
25. Chugh C, Nyirjesy SC, Nawalinski KP, Sandsmark DK, Frangos S, Maloney-Wilensky E. Red Blood Cell Distribution Width is Associated with Poor Clinical Outcome After Subarachnoid Hemorrhage: A Pilot Study. Neurocrit Care. 2015. 23: 217-24
26. Chyatte D, Bruno G, Desai S, Todor DR. Inflammation and intracranial aneurysms. Neurosurgery. 1999. 45: 1137-46
27. Claassen J, Carhuapoma JR, Kreiter KT, Du EY, Connolly ES, Mayer SA. Global cerebral edema after subarachnoid hemorrhage: Frequency, predictors, and impact on outcome. Stroke. 2002. 33: 1225-32
28. Clatterbuck RE, Gailloud P, Ogata L, Gebremariam A, Dietsch GN, Murphy KJ. Prevention of cerebral vasospasm by a humanized anti-CD11/CD18 monoclonal antibody administered after experimental subarachnoid hemorrhage in nonhuman primates. J Neurosurg. 2003. 99: 376-82
29. Clatterbuck RE, Oshiro EM, Hoffman PA, Dietsch GN, Pardoll DM, Tamargo RJ. Inhibition of vasospasm with lymphocyte function-associated antigen-1 monoclonal antibody in a femoral artery model in rats. J Neurosurg. 2002. 97: 676-82
30. Clozel M, Breu V, Burri K, Cassal JM, Fischli W, Gray GA. Pathophysiological role of endothelin revealed by the first orally active endothelin receptor antagonist. Nature. 1993. 365: 759-61
31. Culmsee C, Vedder H, Ravati A, Junker V, Otto D, Ahlemeyer B. Neuroprotection by estrogens in a mouse model of focal cerebral ischemia and in cultured neurons: Evidence for a receptor-independent antioxidative mechanism. J Cereb Blood Flow Metab. 1999. 19: 1263-9
32. Dhar R, Diringer MN. The burden of the systemic inflammatory response predicts vasospasm and outcome after subarachnoid hemorrhage. Neurocrit Care. 2008. 8: 404-12
33. Ding D, Starke RM, Dumont AS, Owens GK, Hasan DM, Chalouhi N. Therapeutic implications of estrogen for cerebral vasospasm and delayed cerebral ischemia induced by aneurysmal subarachnoid hemorrhage. Biomed Res Int 2014. 2014. 727428:
34. El-Etr M, Rame M, Boucher C, Ghoumari AM, Kumar N, Liere P. Progesterone and nestorone promote myelin regeneration in chronic demyelinating lesions of corpus callosum and cerebral cortex. Glia. 2015. 63: 104-17
35. Falkeborn M, Persson I, Terént A, Adami HO, Lithell H, Bergström R. Hormone replacement therapy and the risk of stroke. Follow-up of a population-based cohort in Sweden. Arch Intern Med. 1993. 153: 1201-9
36. Fassbender K, Hodapp B, Rossol S, Bertsch T, Schmeck J, Schütt S. Inflammatory cytokines in subarachnoid haemorrhage: association with abnormal blood flow velocities in basal cerebral arteries. J Neurol Neurosurg Psychiatry. 2001. 70: 534-7
37. Fassbender K, Hodapp B, Rossol S, Bertsch T, Schmeck J, Schütt S. Endothelin-1 in subarachnoid hemorrhage: An acute-phase reactant produced by cerebrospinal fluid leukocytes. Stroke. 2000. 31: 2971-5
38. Feigin VL, Rinkel GJ, Lawes CM, Algra A, Bennett DA, van Gijn J. Risk factors for subarachnoid hemorrhage: An updated systematic review of epidemiological studies. Stroke. 2005. 36: 2773-80
39. Fergus A, Lee KS. Regulation of cerebral microvessels by glutamatergic mechanisms. Brain Res. 1997. 754: 35-45
40. Fountas KN, Tasiou A, Kapsalaki EZ, Paterakis KN, Grigorian AA, Lee GP. Serum and cerebrospinal fluid C-reactive protein levels as predictors of vasospasm in aneurysmal subarachnoid hemorrhage. Clinical article. Neurosurg Focus. 2009. 26: E22-
41. Franco HL, Jeong JW, Tsai SY, Lydon JP, DeMayo FJ. In vivo analysis of progesterone receptor action in the uterus during embryo implantation. Semin Cell Dev Biol. 2008. 19: 178-86
42. Friedrich V, Bederson JB, Sehba FA. Gender influences the initial impact of subarachnoid hemorrhage: An experimental investigation. PLoS One. 2013. 8: e80101-
43. Frontera JA, Provencio JJ, Sehba FA, McIntyre TM, Nowacki AS, Gordon E. The Role of Platelet Activation and Inflammation in Early Brain Injury Following Subarachnoid Hemorrhage. Neurocrit Care. 2017. 26: 48-57
44. Frösen J, Piippo A, Paetau A, Kangasniemi M, Niemelä M, Hernesniemi J. Remodeling of saccular cerebral artery aneurysm wall is associated with rupture: Histological analysis of 24 unruptured and 42 ruptured cases. Stroke. 2004. 35: 2287-93
45. Gaetani P, Tartara F, Pignatti P, Tancioni F, Rodriguez y Baena R. Cisternal CSF levels of cytokines after subarachnoid hemorrhage. Neurol Res. 1998. 20: 337-42
46. Garzon-Muvdi T1, Pradilla G, Ruzevick JJ, Bender M, Edwards L, Grossman R. A glutamate receptor antagonist, S-4-carboxyphenylglycine (S-4-CPG), inhibits vasospasm after subarachnoid hemorrhage in haptoglobin 2-2 mice [corrected]. Neurosurgery. 2013. 73: 719-28
47. Gruber A, Rössler K, Graninger W, Donner A, Illievich MU, Czech T. Ventricular cerebrospinal fluid and serum concentrations of sTNFR-I, IL-1ra, and IL-6 after aneurysmal subarachnoid hemorrhage. J Neurosurg Anesthesiol. 2000. 12: 297-306
48. Gürer B, Turkoglu E, Kertmen H, Karavelioglu E, Arikok AT, Sekerci Z. Attenuation of cerebral vasospasm and secondary injury by testosterone following experimental subarachnoid hemorrhage in rabbit. Acta Neurochir. 2014. 156: 2111-20
49. Hamann G, Isenberg E, Strittmatter M, Schimrigk K. Absence of elevation of big endothelin in subarachnoid hemorrhage. Stroke. 1993. 24: 383-6
50. Hanafy KA, Stuart RM, Khandji AG, Connolly ES, Badjatia N, Mayer SA. Relationship between brain interstitial fluid tumor necrosis factor-alpha and cerebral vasospasm after aneurysmal subarachnoid hemorrhage. J Clin Neurosci. 2010. 17: 853-6
51. Hasan D, Hashimoto T, Kung D, Macdonald RL, Winn HR, Heistad D. Upregulation of cyclooxygenase-2 (COX-2) and microsomal prostaglandin E2 synthase-1 (mPGES-1) in wall of ruptured human cerebral aneurysms: Preliminary results. Stroke. 2012. 43: 1964-7
52. Hasan DM, Chalouhi N, Jabbour P, Magnotta VA, Kung DK, Young WL. Imaging aspirin effect on macrophages in the wall of human cerebral aneurysms using ferumoxytol-enhanced MRI: Preliminary results. J Neuroradiol. 2013. 40: 187-91
53. Hasan DM, Mahaney KB, Brown RD, Meissner I, Piepgras DG, Huston J. Aspirin as a promising agent for decreasing incidence of cerebral aneurysm rupture. Stroke. 2011. 42: 3156-62
54. Hendryk S, Jarzab B, Josko J. Increase of the IL-1 beta and IL-6 levels in CSF in patients with vasospasm following aneurysmal SAH. Neuro Endocrinol Lett. 2004. 25: 141-7
55. Höllig A, Thiel M, Stoffel-Wagner B, Coburn M, Clusmann H. Neuroprotective properties of dehydroepiandrosterone-sulfate and its relationship to interleukin 6 after aneurysmal subarachnoid hemorrhage: A prospective cohort study. Crit Care. 2015. 19: 231-
56. Hopkins SJ, McMahon CJ, Singh N, Galea J, Hoadley M, Scarth S. Cerebrospinal fluid and plasma cytokines after subarachnoid haemorrhage: CSF interleukin-6 may be an early marker of infection. J Neuroinflammation. 2012. 9: 255-
57. Horstmann S, Su Y, Koziol J, Meyding-Lamadé U, Nagel S, Wagner S. MMP-2 and MMP-9 levels in peripheral blood after subarachnoid hemorrhage. J Neurol Sci. 2006. 251: 82-6
58. Hsieh HL, Yang CM. Role of Redox Signaling in Neuroinflammation and Neurodegenerative Diseases. Biomed Res Int 2013. 2013. 484613:
59. Hurn PD, Macrae IM. Estrogen as a neuroprotectant in stroke. J Cereb Blood Flow Metab. 2000. 20: 631-52
60. Hwang SH, Park YS, Kwon JT, Nam TK, Hwang SN, Kang H. Significance of C-reactive protein and transcranial Doppler in cerebral vasospasm following aneurysmal subarachnoid hemorrhage. J Korean Neurosurg Soc. 2013. 54: 289-95
61. Iqbal MJ, Dalton M, Sawers RS. Binding of testosterone and oestradiol to sex hormone binding globulin, human serum albumin and other plasma proteins: Evidence for non-specific binding of oestradiol to sex hormone binding globulin. Clin Sci (Lond). 1983. 64: 307-14
62. Jamous MA, Nagahiro S, Kitazato KT, Satomi J, Satoh K. Role of estrogen deficiency in the formation and progression of cerebral aneurysms. Part I: Experimental study of the effect of oophorectomy in rats. J Neurosurg. 2005. 103: 1046-51
63. Jamous MA, Nagahiro S, Kitazato KT, Tamura T, Kuwayama K, Satoh K. Role of estrogen deficiency in the formation and progression of cerebral aneurysms. Part II: Experimental study of the effects of hormone replacement therapy in rats. J Neurosurg. 2005. 103: 1052-7
64. Jiang Y, Liu DW, Han XY, Dong YN, Gao J, Du B. Neuroprotective effects of anti-tumor necrosis factor-alpha antibody on apoptosis following subarachnoid hemorrhage in a rat model. J Clin Neurosci. 2012. 19: 866-72
65. Jung CS, Lange B, Zimmermann M, Seifert V. The CSF concentration of ADMA, but not of ET-1, is correlated with the occurrence and severity of cerebral vasospasm after subarachnoid hemorrhage. Neurosci Lett. 2012. 524: 20-4
66. Kanematsu Y, Kanematsu M, Kurihara C, Tada Y, Tsou TL, van Rooijen N. Critical roles of macrophages in the formation of intracranial aneurysm. Stroke. 2011. 42: 173-8
67. Kataoka H. Molecular mechanisms of the formation and progression of intracranial aneurysms. Neurol Med Chir (Tokyo). 2015. 55: 214-9
68. Kaynar MY, Tanriverdi T, Kafadar AM, Kacira T, Uzun H, Aydin S. Detection of soluble intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in both cerebrospinal fluid and serum of patients after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2004. 101: 1030-6
69. Kikuchi T, Okuda Y, Kaito N, Abe T. Cytokine production in cerebrospinal fluid after subarachnoid haemorrhage. Neurol Res. 1995. 17: 106-8
70. Kubo Y, Koji T, Yoshida J, Ogawa A, Ogasawara K. Predicting neurological deficit severity due to subarachnoid haemorrhage: Soluble CD40 ligand and platelet-derived growth factor-BB. Crit Care Resusc. 2016. 18: 242-6
71. Kubo Y, Ogasawara K, Kakino S, Kashimura H, Tomitsuka N, Sugawara A. Serum inflammatory adhesion molecules and high-sensitivity C-reactive protein correlates with delayed ischemic neurologic deficits after subarachnoid hemorrhage. Surg Neurol. 2008. 69: 592-6
72. Lenski M, Huge V, Briegel J, Tonn JC, Schichor C, Thon N. Interleukin 6 in the Cerebrospinal Fluid as a Biomarker for Onset of Vasospasm and Ventriculitis After Severe Subarachnoid Hemorrhage. World Neurosurg. 2017. 99: 132-9
73. Leonelli E, Bianchi R, Cavaletti G, Caruso D, Crippa D, Garcia-Segura LM. Progesterone and its derivatives are neuroprotective agents in experimental diabetic neuropathy: A multimodal analysis. Neuroscience. 2007. 144: 1293-304
74. Lin C, Dumont AS, Calisaneller T, Kwan AL, Hwong SL, Lee KS. Monoclonal antibody against E selectin attenuates subarachnoid hemorrhage-induced cerebral vasospasm. Surg Neurol. 2005. 64: 201-5
75. Lin CL, Shih HC, Dumont AS, Kassell NF, Lieu AS, Su YF. The effect of 17beta-estradiol in attenuating experimental subarachnoid hemorrhage-induced cerebral vasospasm. J Neurosurg. 2006. 104: 298-304
76. Lindgren C, Hultin M, Koskinen LO, Lindvall P, Borota L, Naredi S. ADMA levels and arginine/ADMA ratios reflect severity of disease and extent of inflammation after subarachnoid hemorrhage. Neurocrit Care. 2014. 21: 91-101
77. Longstreth WT, Nelson LM, Koepsell TD, van Belle G. Subarachnoid hemorrhage and hormonal factors in women. A population-based case-control study. Ann Intern Med. 1994. 121: 168-73
78. Lu H, Shi JX, Chen HL, Hang CH, Wang HD, Yin HX. Expression of monocyte chemoattractant protein-1 in the cerebral artery after experimental subarachnoid hemorrhage. Brain Res. 2009. 1262: 73-80
79. Macdonald RL. Delayed neurological deterioration after subarachnoid haemorrhage. Nature reviews. Neurology. 2014. 10: 44-58
80. Macdonald RL, Jaja B, Cusimano MD, Etminan N, Hanggi D, Hasan D. SAHIT Investigators--on the outcome of some subarachnoid hemorrhage clinical trials. Translational stroke research. 2013. 4: 286-96
81. Maiuri F, Gallicchio B, Donati P, Carandente M. The blood leukocyte count and its prognostic significance in subarachnoid hemorrhage. J Neurosurg Sci. 1987. 31: 45-8
82. Marbacher S, Marjamaa J, Bradacova K, von Gunten M, Honkanen P, Abo-Ramadan U. Loss of mural cells leads to wall degeneration, aneurysm growth, and eventual rupture in a rat aneurysm model. Stroke. 2014. 45: 248-54
83. Masaoka H, Suzuki R, Hirata Y, Emori T, Marumo F, Hirakawa K. Raised plasma endothelin in aneurysmal subarachnoid haemorrhage. Lancet. 1989. 2: 1402-
84. Mascia L, Fedorko L, Stewart DJ, Mohamed F, terBrugge K, Ranieri VM. Temporal relationship between endothelin-1 concentrations and cerebral vasospasm in patients with aneurysmal subarachnoid hemorrhage. Stroke. 2001. 32: 1185-90
85. Matsumoto A. Hormonally induced neuronal plasticity in the adult motoneurons. Brain Res Bull. 1997. 44: 539-47
86. McGirt MJ, Mavropoulos JC, McGirt LY, Alexander MJ, Friedman AH, Laskowitz DT. Leukocytosis as an independent risk factor for cerebral vasospasm following aneurysmal subarachnoid hemorrhage. J Neurosurg. 2003. 98: 1222-6
87. McMahon CJ, Hopkins S, Vail A, King AT, Smith D, Illingworth KJ. Inflammation as a predictor for delayed cerebral ischemia after aneurysmal subarachnoid haemorrhage. J Neurointerv Surg. 2013. 5: 512-7
88. Mhurchu CN, Anderson C, Jamrozik K, Hankey G, Dunbabin D. Hormonal factors and risk of aneurysmal subarachnoid hemorrhage: An international population-based, case-control study. Stroke. 2001. 32: 606-12
89. Miller BA, Turan N, Chau M, Pradilla G. Inflammation, vasospasm, and brain injury after subarachnoid hemorrhage. Biomed Res Int 2014. 2014. 384342:
90. Mocco J, Mack WJ, Kim GH, Lozier AP, Laufer I, Kreiter KT. Rise in serum soluble intercellular adhesion molecule-1 levels with vasospasm following aneurysmal subarachnoid hemorrhage. J Neurosurg. 2002. 97: 537-41
91. Moreno P, Beisken S, Harsha B, Muthukrishnan V, Tudose I, Dekker A. BiNChE: A web tool and library for chemical enrichment analysis based on the ChEBI ontology. BMC Bioinformatics. 2015. 16: 56-
92. Morley P, Small DL, Murray CL, Mealing GA, Poulter MO, Durkin JP. Evidence that functional glutamate receptors are not expressed on rat or human cerebromicrovascular endothelial cells. J Cereb Blood Flow Metab. 1998. 18: 396-406
93. Muroi C, Seule M, Sikorski C, Dent W, Keller E. Systemic interleukin-6 levels reflect illness course and prognosis of patients with spontaneous nonaneurysmal subarachnoid hemorrhage. Acta Neurochir Suppl. 2013. 115: 77-80
94. Murthy SB, Naval NS. Dehydroepiandrosterone sulphate: diabolical hormone or epiphenomenon in aneurysmal subarachnoid hemorrhage?. Crit Care. 2015. 19: 352-
95. Nam DH, Kim JS, Hong SC, Lee WH, Lee JI, Shin HJ. Expression of interleukin-1 beta in lipopolysaccharide stimulated monocytes derived from patients with aneurysmal subarachnoid hemorrhage is correlated with cerebral vasospasm. Neurosci Lett. 2001. 312: 41-4
96. Neil-Dwyer G, Cruickshank J. The blood leucocyte count and its prognostic significance in subarachnoid haemorrhage. Brain. 1974. 97: 79-86
97. Ni W, Gu YX, Song DL, Leng B, Li PL, Mao Y. The relationship between IL-6 in CSF and occurrence of vasospasm after subarachnoid hemorrhage. Acta Neurochir Suppl. 2011. 110: 203-8
98. Niikawa S, Hara S, Ohe N, Miwa Y, Ohkuma A. Correlation between blood parameters and symptomatic vasospasm in subarachnoid hemorrhage patients. Neurol Med Chir. 1997. 37: 881-4
99. Obata Y, Takeda J, Sato Y, Ishikura H, Matsui T, Isotani E. A multicenter prospective cohort study of volume management after subarachnoid hemorrhage: Circulatory characteristics of pulmonary edema after subarachnoid hemorrhage. J Neurosurg. 2016. 125: 254-63
100. Oliveira-Filho J, Ezzeddine MA, Segal AZ, Buonanno FS, Chang Y, Ogilvy CS. Fever in subarachnoid hemorrhage: relationship to vasospasm and outcome. Neurology. 2001. 56: 1299-304
101. Oshiro EM1, Hoffman PA, Dietsch GN, Watts MC, Pardoll DM, Tamargo RJ. Inhibition of experimental vasospasm with anti-intercellular adhesion molecule-1 monoclonal antibody in rats. Stroke. 1997. 28: 2031-7
102. Osuka K, Suzuki Y, Tanazawa T, Hattori K, Yamamoto N, Takayasu M. Interleukin-6 and development of vasospasm after subarachnoid haemorrhage. Acta Neurochir (Wien). 1998. 140: 943-51
103. Pedersen AT, Lidegaard O, Kreiner S, Ottesen B. Hormone replacement therapy and risk of non-fatal stroke. Lancet. 1997. 350: 1277-83
104. Pellettieri L, Nilsson B, Carlsson CA, Nilsson U. Serum immunocomplexes in patients with subarachnoid hemorrhage. Neurosurgery. 1986. 19: 767-71
105. Peterson BL, Won S, Geddes RI, Sayeed I, Stein DG. Sex-related differences in effects of progesterone following neonatal hypoxic brain injury. Behav Brain Res. 2015. 286: 152-65
106. Pike CJ. Testosterone attenuates beta-amyloid toxicity in cultured hippocampal neurons. Brain Res. 2001. 919: 160-5
107. Polin RS, Bavbek M, Shaffrey ME, Billups K, Bogaev CA, Kassell NF. Detection of soluble E-selectin, ICAM-1, VCAM-1, and L-selectin in the cerebrospinal fluid of patients after subarachnoid hemorrhage. J Neurosurg. 1998. 89: 559-67
108. Pradilla G, Garzon-Muvdi T, Ruzevick JJ, Bender M, Edwards L, Momin EN. Systemic L-citrulline prevents cerebral vasospasm in haptoglobin 2-2 transgenic mice after subarachnoid hemorrhage. Neurosurgery. 2012. 70: 747-56
109. Pradilla G, Wang PP, Legnani FG, Ogata L, Dietsch GN, Tamargo RJ. Prevention of vasospasm by anti-CD11/CD18 monoclonal antibody therapy following subarachnoid hemorrhage in rabbits. J Neurosurg. 2004. 101: 88-92
110. Provencio JJ, Swank V, Lu H, Brunet S, Baltan S, Khapre RV. Neutrophil depletion after subarachnoid hemorrhage improves memory via NMDA receptors. Brain Behav Immun. 2016. 54: 233-42
111. Ramchand P, Nyirjesy S, Frangos S, Doerfler S, Nawalinski K, Quattrone F. Thromboelastography Parameter Predicts Outcome After Subarachnoid Hemorrhage: An Exploratory Analysis. World Neurosurg. 2016. 96: 215-21
112. Rattanajarasroj S, Unchern S. Comparable attenuation of Abeta (25-35)-induced neurotoxicity by quercitrin and 17beta-estradiol in cultured rat hippocampal neurons. Neurochem Res. 2010. 35: 1196-205
113. Recinos PF, Pradilla G, Thai QA, Perez M, Hdeib AM, Tamargo RJ. Controlled release of lipopolysaccharide in the subarachnoid space of rabbits induces chronic vasospasm in the absence of blood. Surg Neurol. 2006. 66: 463-9
114. Rodling-Wahlström M, Olivecrona M, Koskinen LO, Naredi S, Hultin M. Subarachnoid haemorrhage induces an inflammatory response followed by a delayed persisting increase in asymmetric dimethylarginine. Scand J Clin Lab Invest. 2012. 72: 484-9
115. Rousseaux P, Scherpereel B, Bernard MH, Graftieaux JP, Guyot JF. Fever and cerebral vasospasm in ruptured intracranial aneurysms. Surg Neurol. 1980. 14: 459-65
116. Sabri M, Ai J, Knight B, Tariq A, Jeon H, Shang X, Marsden PA. Uncoupling of endothelial nitric oxide synthase after experimental subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2011. 31: 190-9
117. Savarraj J, Parsha K, Hergenroeder G, Ahn S, Chang TR, Kim DH. Early Brain Injury Associated with Systemic Inflammation After Subarachnoid Hemorrhage. Neurocrit Care. 2017. p.
118. Sayeed I, Stein DG. Progesterone as a neuroprotective factor in traumatic and ischemic brain injury. Prog Brain Res. 2009. 175: 219-37
119. Schumacher M, Mattern C, Ghoumari A, Oudinet JP, Liere P, Labombarda F4. Revisiting the roles of progesterone and allopregnanolone in the nervous system: Resurgence of the progesterone receptors. Prog Neurobiol. 2014. 113: 6-39
120. Seifert V, Löffler BM, Zimmermann M, Roux S, Stolke D. Endothelin concentrations in patients with aneurysmal subarachnoid hemorrhage. Correlation with cerebral vasospasm, delayed ischemic neurological deficits, and volume of hematoma. J Neurosurg. 1995. 82: 55-62
121. Shi C, Awad IA, Jafari N, Lin S, Du P, Hage ZA. Genomics of human intracranial aneurysm wall. Stroke. 2009. 40: 1252-61
122. Singer CA, Figueroa-Masot XA, Batchelor RH, Dorsa DM. The mitogen-activated protein kinase pathway mediates estrogen neuroprotection after glutamate toxicity in primary cortical neurons. J Neurosci. 1999. 19: 2455-63
123. Singh TD, Maloney P, Rabinstein AA, Hocker S. Significance of routine cerebrospinal fluid analysis in subarachnoid hemorrhage. J Neurosurg Sci. 2017. 61: 117-23
124. Sozen T, Tsuchiyama R, Hasegawa Y, Suzuki H, Jadhav V, Nishizawa S. Immunological response in early brain injury after SAH. Acta Neurochir Suppl. 2011. 110: 57-61
125. Spallone A, Acqui M, Pastore FS, Guidetti B. Relationship between leukocytosis and ischemic complications following aneurysmal subarachnoid hemorrhage. Surg Neurol. 1987. 27: 253-8
126. Srinivasan A, Aggarwal A, Gaudihalli S, Mohanty M, Dhandapani M, Singh H. Impact of Early Leukocytosis and Elevated High-Sensitivity C-Reactive Protein on Delayed Cerebral Ischemia and Neurologic Outcome After Subarachnoid Hemorrhage. World Neurosurg. 2016. 90: 91-5
127. Stein DG. Embracing failure: What the Phase III progesterone studies can teach about TBI clinical trials. Brain Injury. 2015. 29: 1259-72
128. Sun X, Ji C, Hu T, Wang Z, Chen G. Tamoxifen as an effective neuroprotectant against early brain injury and learning deficits induced by subarachnoid hemorrhage: possible involvement of inflammatory signaling. J Neuroinflammation. 2013. 10: 157-
129. Tada Y, Makino H, Furukawa H, Shimada K, Wada K, Liang EI. Roles of estrogen in the formation of intracranial aneurysms in ovariectomized female mice. Neurosurgery. 2014. 75: 690-5
130. Tam AK, Ilodigwe D, Mocco J, Mayer S, Kassell N, Ruefenacht D. Impact of systemic inflammatory response syndrome on vasospasm, cerebral infarction, and outcome after subarachnoid hemorrhage: exploratory analysis of CONSCIOUS-1 database. Neurocrit Care. 2010. 13: 182-9
131. Thomas P, Pang Y. Membrane progesterone receptors: evidence for neuroprotective, neurosteroid signaling and neuroendocrine functions in neuronal cells. Neuroendocrinology. 2012. 96: 162-71
132. Tulamo R, Frösen J, Junnikkala S, Paetau A, Kangasniemi M, Peláez J. Complement system becomes activated by the classical pathway in intracranial aneurysm walls. Lab Invest. 2010. 90: 168-79
133. Turan N, Heider RA, Zaharieva D, Ahmad FU, Barrow DL, Pradilla G. Sex Differences in the Formation of Intracranial Aneurysms and Incidence and Outcome of Subarachnoid Hemorrhage: Review of Experimental and Human Studies. Transl Stroke Res. 2016. 7: 12-9
134. Turan N, Miller BA, Heider RA, Nadeem M, Sayeed I, Stein DG. Neurobehavioral testing in subarachnoid hemorrhage: A review of methods and current findings in rodents. J Cereb Blood Flow Metab. 2017. 37: 3461-3474
135. Turan N, Miller BA, Huie JR, Heider RA, Wang J, Wali B. Effect of Progesterone on Cerebral Vasospasm and Neurobehavioral Outcomes in a Rodent Model of Subarachnoid Hemorrhage. World Neurosurg. 2018. 110: e150-e159
136. Vedder H, Anthes N, Stumm G, Würz C, Behl C, Krieg JC. Estrogen hormones reduce lipid peroxidation in cells and tissues of the central nervous system. J Neurochem. 1999. 72: 2531-8
137. Wali B, Ishrat T, Stein DG, Sayeed I. Progesterone improves long-term functional and histological outcomes after permanent stroke in older rats. Behav Brain Res. 2016. 305: 46-56
138. Wali B, Sayeed I, Guthrie DB, Natchus MG, Turan N, Liotta DC. Evaluating the neurotherapeutic potential of a water-soluble progesterone analog after traumatic brain injury in rats. Neuropharmacology. 2016. 109: 148-58
139. Walton JN. The prognosis and management of subarachnoid haemorrhage. Can Med Assoc J. 1955. 72: 165-75
140. Wang Y, Zhong M, Tan XX, Yang YJ, Chen WJ, Liu W. Expression change of interleukin-8 gene in rabbit basilar artery after subarachnoid hemorrhage. Neurosci Bull. 2007. 23: 151-5
141. Wang Z, Zuo G, Shi XY, Zhang J, Fang Q, Chen G. Progesterone administration modulates cortical TLR4/NF-kappaB signaling pathway after subarachnoid hemorrhage in male rats. Mediators Inflamm 2011. 2011. 848309:
142. Wartenberg KE, Mayer SA. Medical complications after subarachnoid hemorrhage. Neurosurg Clin N Am. 2010. 21: 325-38
143. Webster KM, Wright DK, Sun M, Semple BD, Ozturk E, Stein DG. Progesterone treatment reduces neuroinflammation, oxidative stress and brain damage and improves long-term outcomes in a rat model of repeated mild traumatic brain injury. J Neuroinflammation. 2015. 12: 238-
144. Weir B, Disney L, Grace M, Roberts P. Daily trends in white blood cell count and temperature after subarachnoid hemorrhage from aneurysm. Neurosurgery. 1989. 25: 161-5
145. Wu W, Guan Y, Zhao G, Fu XJ, Guo TZ, Liu YT. Elevated IL-6 and TNF-alpha Levels in Cerebrospinal Fluid of Subarachnoid Hemorrhage Patients. Mol Neurobiol. 2016. 53: 3277-85
146. Wunderle K, Hoeger KM, Wasserman E, Bazarian JJ. Menstrual phase as predictor of outcome after mild traumatic brain injury in women. J Head Trauma Rehabil. 2014. 29: E1-8
147. Xie X, Wu X, Cui J, Li H, Yan X. Increase ICAM-1 and LFA-1 expression by cerebrospinal fluid of subarachnoid hemorrhage patients: Involvement of TNF-alpha. Brain Res. 2013. 1512: 89-96
148. Yamaura I, Tani E, Maeda Y, Minami N, Shindo H. Endothelin-1 of canine basilar artery in vasospasm. J Neurosurg. 1992. 76: 99-105
149. Yan F, Hu Q, Chen J, Wu C, Gu C, Chen G. Progesterone attenuates early brain injury after subarachnoid hemorrhage in rats. Neurosci Lett. 2013. 543: 163-7
150. Yang YM, Huang A, Kaley G, Sun D. eNOS uncoupling and endothelial dysfunction in aged vessels. Am J Physiol Heart Circ Physiol. 2009. 297: H1829-36
151. Yeung PK, Shen J, Chung SS, Chung SK. Targeted over-expression of endothelin-1 in astrocytes leads to more severe brain damage and vasospasm after subarachnoid hemorrhage. BMC Neurosci. 2013. 14: 131-
152. Young AM, Karri SK, Ogilvy CS. Exploring the use of estrogen & progesterone replacement therapy in subarachnoid hemorrhage. Curr Drug Saf. 2012. 7: 202-6
153. Zanier ER, Zangari R, Munthe-Fog L, Hein E, Zoerle T, Conte V. Ficolin-3-mediated lectin complement pathway activation in patients with subarachnoid hemorrhage. Neurology. 2014. 82: 126-34
154. Zhang Z, Liu J, Fan C, Mao L, Xie R, Wang S. The GluN1/GluN2B NMDA receptor and metabotropic glutamate receptor 1 negative allosteric modulator has enhanced neuroprotection in a rat subarachnoid hemorrhage model. Exp Neurol. 2018. 301: 13-25
155. Zhao XD, Zhou YT. Effects of progesterone on intestinal inflammatory response and mucosa structure alterations following SAH in male rats. J Surg Res. 2011a. 171: e47-53
156. Zhao XD, Zhou YT. Effects of progesterone on intestinal inflammatory response and mucosa structure alterations following SAH in male rats. J Surg Res. 2011b. 171: e47-53
157. Zhong W, Zhang Z, Zhao P, Shen J, Li X, Wang D, Li G. The Impact of Initial Systemic Inflammatory Response After Aneurysmal Subarachnoid Hemorrhage. Turk Neurosurg. 2017. 27: 346-52
158. Zuccarello M, Boccaletti R, Romano A, Rapoport RM. Endothelin B receptor antagonists attenuate subarachnoid hemorrhage-induced cerebral vasospasm. Stroke. 1998. 29: 1924-9