- Department of Neurosurgery, School of Medicine, Fukuoka University Hospital, Fukuoka, Japan.
- Department of Neurosurgery, Fukuoka Chikushi Hospital, Fukuoka, Japan.
Correspondence Address:
Kenji Fukuda
Department of Neurosurgery, School of Medicine, Fukuoka University Hospital, Fukuoka, Japan.
DOI:10.25259/SNI_565_2020
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Dai Kawano1, Kenji Fukuda1, Hironori Fukumoto1, Yoshinobu Horio1, Masaki Takahara1, Hiroshi Abe1, Toshio Higashi2, Tooru Inoue1. The usefulness of temporary balloon occlusion during transarterial embolization for scalp arteriovenous fistula. 08-Mar-2021;12:89
How to cite this URL: Dai Kawano1, Kenji Fukuda1, Hironori Fukumoto1, Yoshinobu Horio1, Masaki Takahara1, Hiroshi Abe1, Toshio Higashi2, Tooru Inoue1. The usefulness of temporary balloon occlusion during transarterial embolization for scalp arteriovenous fistula. 08-Mar-2021;12:89. Available from: https://surgicalneurologyint.com/surgicalint-articles/10634/
Abstract
Background: We present two cases of scalp arteriovenous fistula (sAVF) treated by transarterial embolization with the aid of a temporary balloon occlusion (TBO) to detect precise vasculature.
Case Description: Case 1: A 59-year-old woman noticed a sudden onset of pulsating bruits. sAVF was fed by the right superficial temporal artery (STA) and drained to the right superficial temporal vein. We performed feeder occlusion using coils after the recognition of a single feeder and a single fistula using TBO. Case 2: A 42-year-old woman noticed a pulsating subcutaneous mass. sAVF was fed by the right occipital artery (OA) and drained to the right occipital vein (OV). We could detect another feeder of the right STA after the TBO of the OA. We performed transarterial feeder occlusion for STA and OA using coil and N-butyl-2-cyanoacrylat including OV and shunt point, because this case was a single fistula with multiple feeders.
Conclusion: sAVFs are a relatively rare disease with a complex vascular structure. For the adequate transarterial approach, TBO was useful for detecting the precise vasculature of sAVF.
Keywords: Scalp arteriovenous fistula, Temporary balloon occlusion, Transarterial embolization
INTRODUCTION
Scalp arteriovenous fistulas (sAVFs) are rare abnormal connections between subcutaneous arteries and veins without an intervening capillary bed. They are also referred to as cirsoid aneurysm, aneurysma serpentinum, aneurysm racemosum, or plexiform angioma.[
Treatment includes surgical excision, endovascular embolization, or a combination of these treatments.[
CASE REPORT
Case 1
The patient was a 53-year-old woman with a history of hyperlipidemia but no past episode of trauma. The patient noticed a sudden onset of pulsating bruits in the right preauricular region. The symptoms did not improve over 2 years. Therefore, the patient underwent MRI at another hospital and was referred to our hospital for suspected sAVF. On admission, no neurological abnormalities were noted. We noticed pulsating bruits, thrill at the right preauricular region. CT angiography (CTA) [
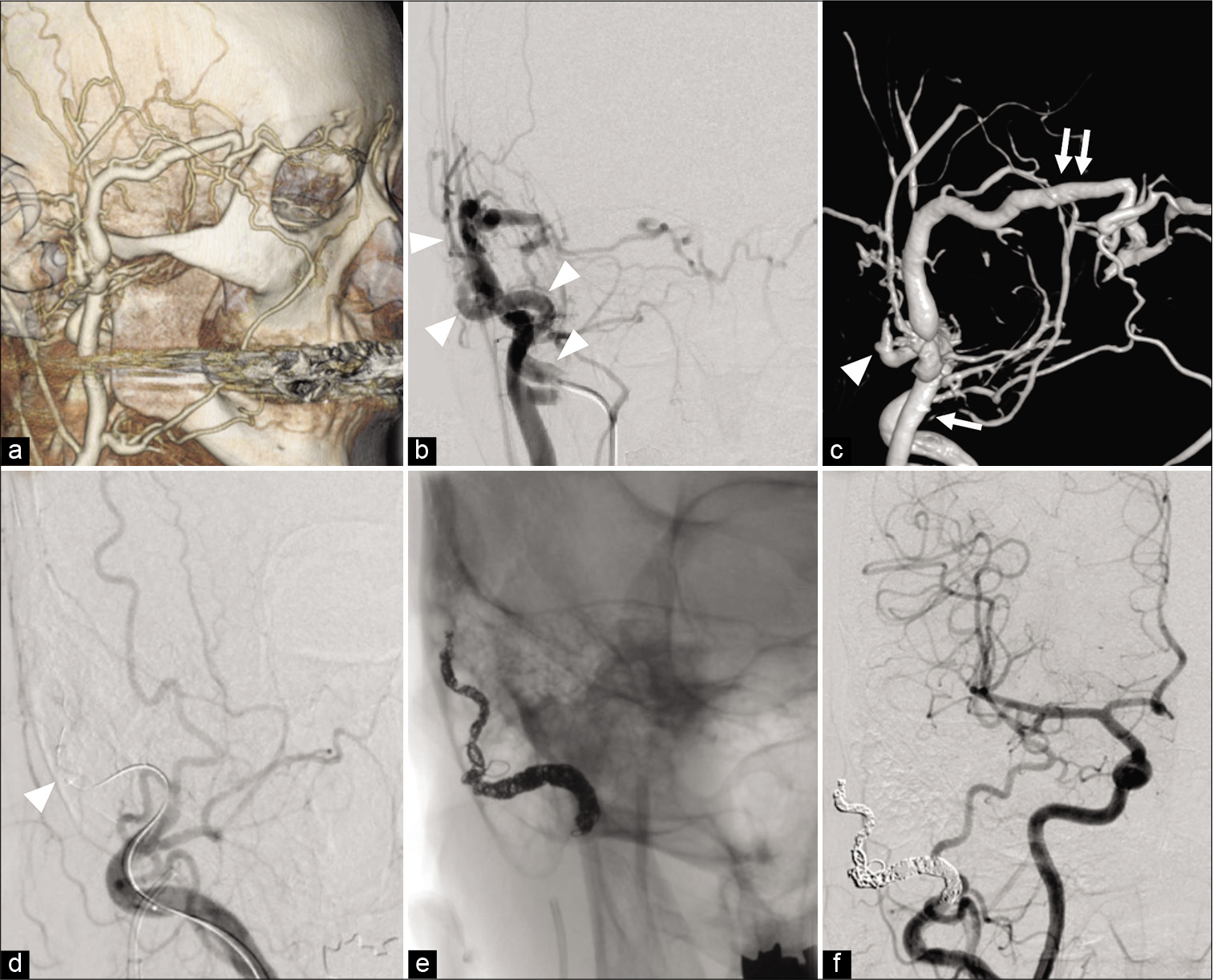
Figure 1:
(a) CT angiography showing the dilated subcutaneous vein suspected the existence of an arteriovenous shunt in the right preauricular region. (b) DSA showing scalp arteriovenous fistula with a fistula at the right preauricular region fed by the right STA (arrowhead). (c) 3D-DSA showing the fistulous point (arrowhead) is located in the preauricular region fed by the right STA and drained to the right STV to lower the external jugular vein (arrow) and to the superior ophthalmic vein through the angular vein (double arrow). (d). TBO using Hyperform 4 mm × 7 mm (Medtronic, Minneapolis, MN, USA) placed in the STA just proximal to the fistulous point (arrowhead) reveals that no additional feeder exists. (e) Transarterial embolization for STA was performed using platinum coils. (f) The disappearance of the arteriovenous shunt is confirmed. sAVF: Scalp arteriovenous fistula, TBO: temporary balloon occlusion, ST: Superficial temporal artery, OA: Occipital artery, OV: Occipital vein, NBCA: N-butyl-2-cyanoacrylat, DSA: Digital subtraction angiography, CTA: CT angiography.
Case 2
The patient was a 42-year-old woman with no history of traumatic episodes. She noticed a pulsating subcutaneous mass in the right occipital head. The patient underwent CTA at a local plastic surgery hospital and for suspected sAVF and was referred to our hospital. On admission, no neurological abnormalities were noted. We noticed a pulsating mass and bruit of the right back of the head. CTA [
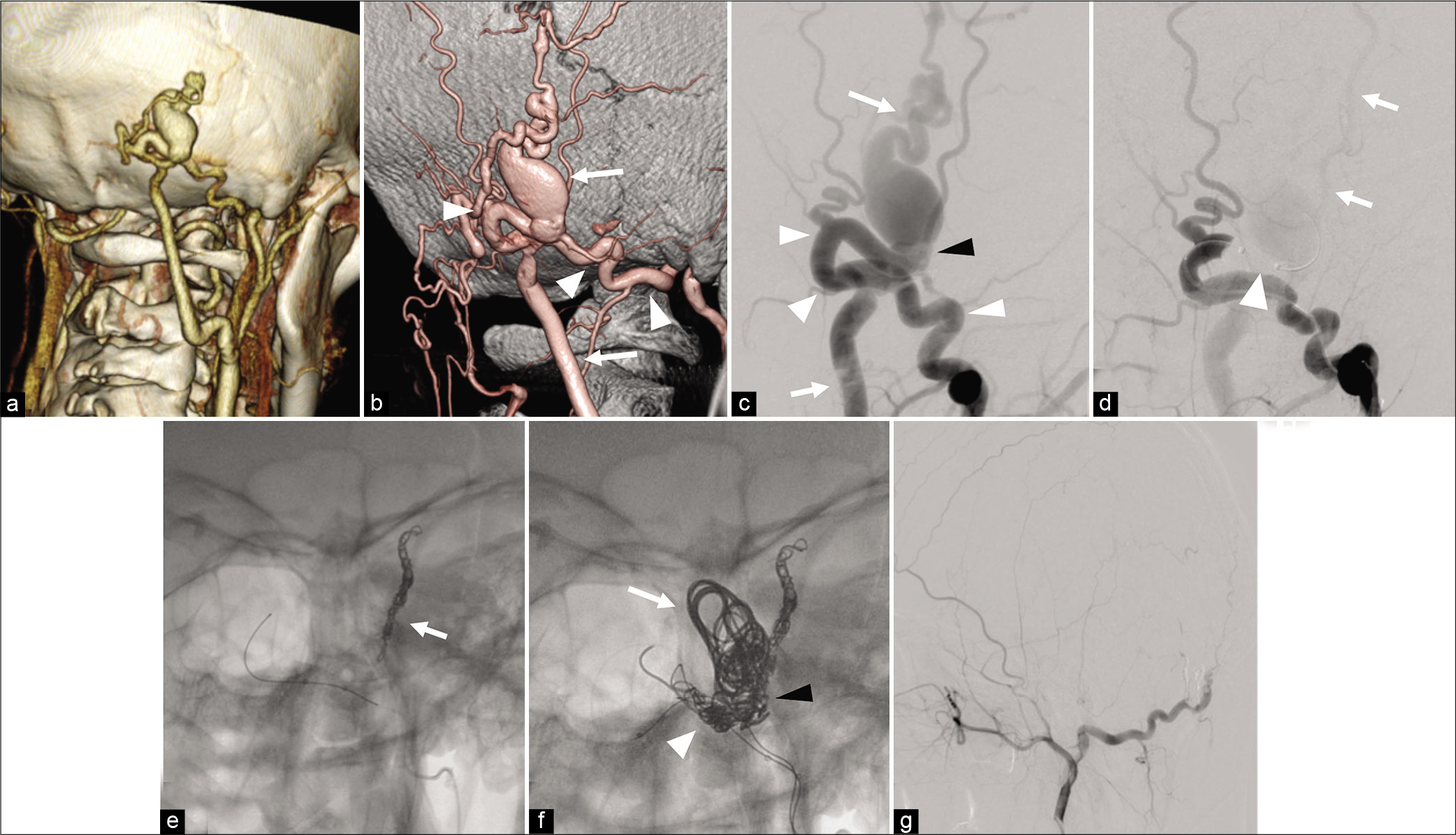
Figure 2:
(a) CT angiography showing the dilated subcutaneous vein suspected the existence of an arteriovenous shunt in the right occipital head. (b) 3D-DSA showing scalp arteriovenous fistula with a fistula at the right occipital head fed by the right OA (arrowhead) and drained to the right OV (arrow). (c) DSA showing the OA and OV has a direct connection at the fistulous point (black arrowhead). (d) TBO using SHORYU HR 4 mm × 7 mm (Kaneka Medix Corp, Osaka, Japan) (arrowhead) placed in the OA just proximal to the fistulous point (arrowhead) reveals that an additional feeder from the STA (arrow) with retrograde flow exists. (e) TAE for STA was performed using coils and NBCA. (f) TAE for dilated OV (arrow) including shunt point (black arrowhead), and OA (arrowhead) using coil and NBCA. (g) The disappearance of the arteriovenous shunt is confirmed. sAVF: Scalp arteriovenous fistula, TBO: temporary balloon occlusion, ST: Superficial temporal artery, OA: Occipital artery, OV: Occipital vein, NBCA: N-butyl-2-cyanoacrylat, DSA: Digital subtraction angiography, CTA: CT angiography.
DISCUSSION
sAVFs are rare subcutaneous diseases with abnormal connections between subcutaneous arteries and veins without an intervening capillary bed. In a recent systematic review of the literature, Sofela et al. reported that the etiology of sAVF was that 60.2% were spontaneous, 32.3% trauma, and 7.5% iatrogenic in total 74 patients.[
They can be managed by surgical excision only, endovascular embolization only, or a combination of both.[
The transarterial approach is easier compared with the transvenous approach especially when the target vessel is dilated and less tortuous.[
CONCLUSION
sAVFs are rare diseases with complex vasculature. For the safe and effective endovascular transarterial approach for sAVF, TBO is useful for understanding the precise vasculature.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We acknowledge the assistance of editorial services that provided language.
References
1. Arat A, Cil BE, Vargel I, Turkbey B, Canyigit M, Peynircioglu B. Embolization of high-flow craniofacial vascular malformations with onyx. AJNR Am J Neuroradiol. 2007. 28: 1409-14
2. Dalyai RT, Schirmer CM, Malek AM. Transvenous balloon-protected embolization of a scalp arteriovenous fistula using onyx liquid embolic. Acta Neurochir (Wien). 2011. 153: 1285-90
3. Fisher-Jeffes ND, Domingo Z, Madden M, de Villiers JC. Arteriovenous malformations of the scalp. Neurosurgery. 1995. 36: 656-60
4. Komiyama M, Nishikawa M, Kitano S, Sakamoto H, Imai K, Tsujiguchi K. Non-traumatic arteriovenous fistulas of the scalp treated by a combination of embolization and surgical removal. Neurol Med Chir (Tokyo). 1996. 36: 162-5
5. Li D, Heiferman DM, Rothstein BD, Syed HR, Shaibani A, Tomita T. Scalp arteriovenous malformation (cirsoid aneurysm) in adolescence: Report of 2 cases and review of the literature. World Neurosurg. 2018. 116: e1042-6
6. Ni W, Tian Y, Gu Y, Mao Y. Transvenous endovascular treatment for scalp arteriovenous fistulas: Results with combined use of onyx and coils. World Neurosurg. 2017. 107: 692-7
7. Senoglu M, Yasim A, Gokce M, Senoglu N. Nontraumatic scalp arteriovenous fistula in an adult: Technical report on an illustrative case. Surg Neurol. 2008. 70: 194-7
8. Sofela A, Osunronbi T, Hettige S. Scalp cirsoid aneurysms: Case illustration and systematic review of literature. Neurosurgery. 2020. 86: E98-107
9. Sousa LH, Gatto LA, Junior Z, Koppe GL. Scalp cirsoid aneurysm: An updated systematic literature review and an illustrative case report. World Neurosurg. 2018. 119: 416-27
10. Yokouchi T, Iwabuchi S, Tomiyama A, Samejima H, Ogata N, Goto K. Embolization of scalp AVF. Interv Neuroradiol. 1999. 5: 121-6