- Department of Neuro-Oncology, Aurora Cancer Care, Milwaukee, Wisconsin, United States,
- Department of Pathology, Baylor University Medical Center, Dallas, Texas, United States,
- Department of Neurology, Neuro-Oncology Unit, Instituto Neurológico de Colombia/Universidad CES, Medellín, Antioquia, Colombia.
Correspondence Address:
Esteban Jaramillo-Jiménez
Department of Neurology, Neuro-Oncology Unit, Instituto Neurológico de Colombia/Universidad CES, Medellín, Antioquia, Colombia.
DOI:10.25259/SNI_533_2019
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Isaac Melguizo-Gavilanes, George Snipes, Iader Rodríguez-Márquez, Laura Duarte-Jurado, Esteban Jaramillo-Jiménez. Therapeutic options for primary meningeal angiosarcoma: A case report. 25-Jul-2020;11:204
How to cite this URL: Isaac Melguizo-Gavilanes, George Snipes, Iader Rodríguez-Márquez, Laura Duarte-Jurado, Esteban Jaramillo-Jiménez. Therapeutic options for primary meningeal angiosarcoma: A case report. 25-Jul-2020;11:204. Available from: https://surgicalneurologyint.com/surgicalint-articles/10160/
Abstract
Background: Primary angiosarcoma (AS) of the central nervous system (PACNS) is an extremely rare malignancy. The meninges represent an uncommon site of origin of PACNS. This report describes a recurrent meningeal PACNS treated with surgery, radiotherapy, stereotactic radiosurgery, and paclitaxel at different stages of the disease.
Case Description: A 36-year-old Asian male presented to our facility with a 4-month history of worsening headaches and complete right homonymous hemianopia. Neuroimaging revealed a left occipital lobe hematome with an underlying left tentorial tumor. After subtotal resection, neuropathological examination revealed features of a malignant endothelial cell AS. He received a course of adjuvant radiation therapy but experienced disease progression. He subsequently received additional stereotactic radiosurgery followed by weekly paclitaxel. Magnetic resonance imaging during the course of treatment revealed stable disease until patient died following another progression of his tumor.
Conclusion: This case of a meningeal PACNS highlights the importance of considering this entity in the face of a malignant lesion presenting with intracranial hemorrhagic activity. Our observations suggest that the use of paclitaxel provided a modest clinical response in PACNS, highlighting the need to consider a combined approach structured mainly on surgery and radiotherapy. Stereotactic radiosurgery appears to be a promising treatment option.
Keywords: Hemangiosarcoma, Meninges, Vascular neoplasms
INTRODUCTION
Angiosarcomas (AS) are malignant vascular neoplasms representing <1% of all sarcomas.[
CASE DESCRIPTION
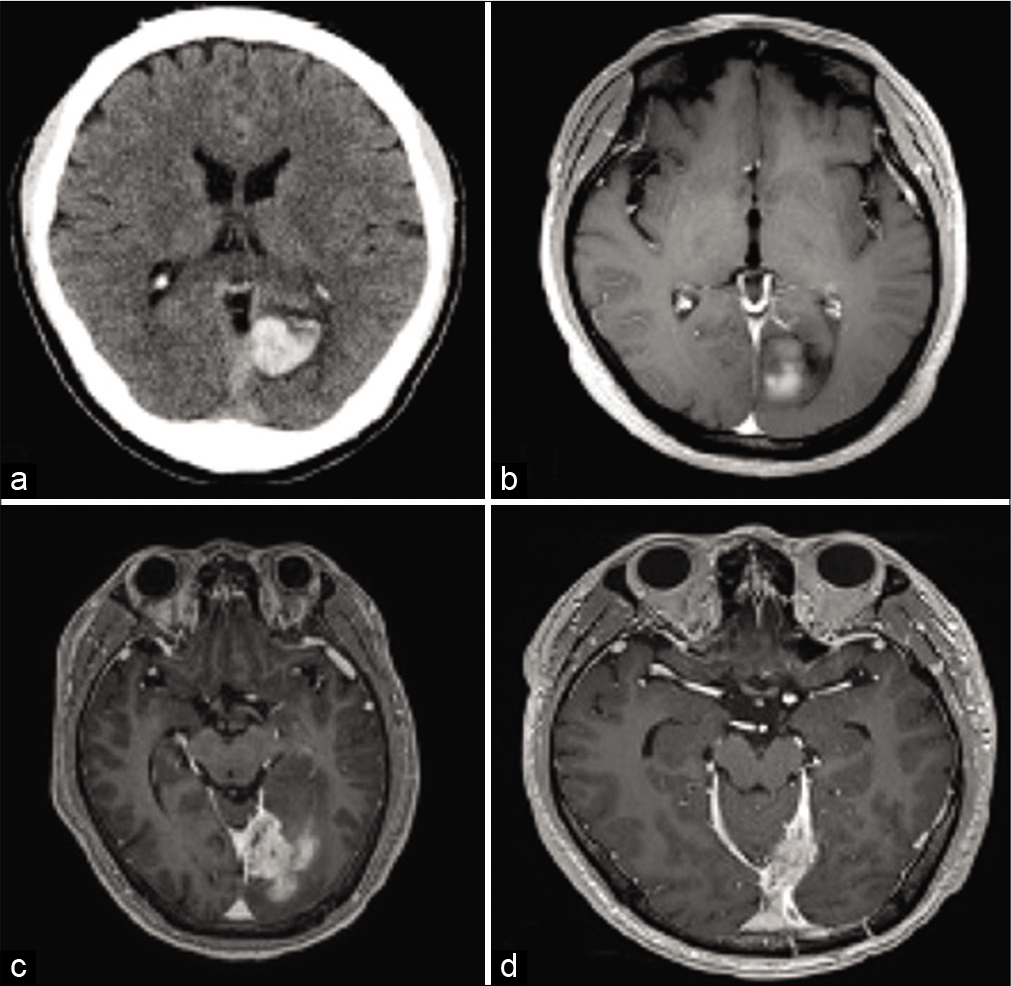
A 36-year-old Asian male presented to our facility with a 4-month history of worsening headaches and complete right homonymous hemianopia. He had a medical background of the left occipital, intracranial cerebral hematoma 2 years before the current event, the etiology of which was never found. The ensuing computed tomography (CT) and magnetic resonance imaging (MRI) scans revealed a left occipital lobe hematoma with an underlying left tentorial tumor, suggesting the presence of a cavernous angioma [
Figure 1:
Initial presentation of a left occipital lobe intraparenchymal hemorrhage. (a) Head computed tomography without contrast. (b) Axial T1-weighted magnetic resonance imaging (MRI) with contrast. (c) Axial T1-weighted MRI with contrast revealing a left tentorial extra-axial enhancing mass with parenchymal hemorrhage. (d) Baseline axial T1 with contrast before initiation of paclitaxel.
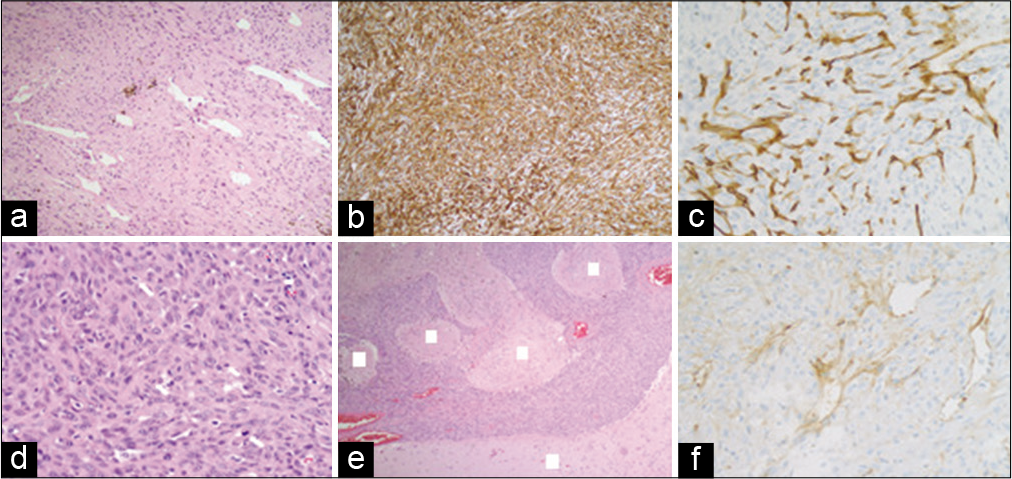
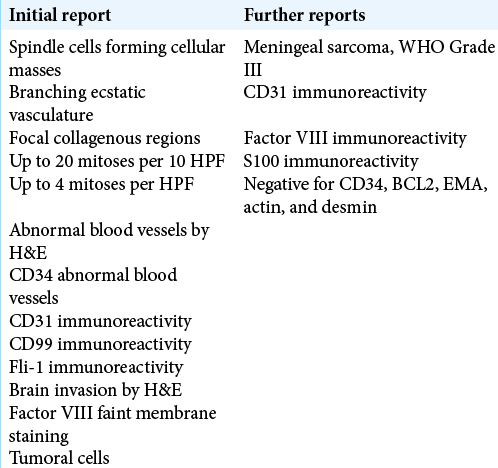
After maximal save resection (subtotal resection), the histopathological examination demonstrated the presence of spindle cells forming masses with branching ecstatic vasculature and focal collagenous regions, with up to 20 mitoses per ten high power fields. Further immunohistochemical studies showed strong immunoreactivity for CD31, CD99, and Fli-1 with a few scattered S100 positive cells but negative for CD34, EMA, desmin, muscle-specific actin, and Bcl-2; the latter findings were suggestive of meningeal sarcoma with angiosarcomatous features, ultimately assessed as a malignant endothelial cell sarcoma/AS [
Nevertheless, in view of the complex morphology, location and patterns of infiltration of this tumor, further efforts were made toward a broad differential diagnostic panel. We screened our patient with a next generation sequencing platform performed at Foundation Medicine (Cambridge, USA). Two diagnostic panels were performed (FoundationOne and Foundation heme assay), analyzing 236 genes, 47 introns from 19 genes rearrangements for solid tumors including non-small cell lung cancer, colorectal, breast, ovarian, and Melanoma, and 405 DNA genes, 31 introns from rearrangements, 265 RNA genes, gene fusion for hematologic tumors including leukemias, myelodysplastic syndrome, myeloproliferative neoplasm, acute myeloid leukemia, and acute lymphoblastic leukemia, respectively. Both of these tests were negative for identifying any genomic alterations with therapeutic implications. Gliosarcoma, a new variant of IDH-wildtype glioblastoma based on the WHO 2016 classification characterized by a biphasic tissue pattern with alternating areas displaying glial and mesenchymal differentiation was also considered. Further molecular analysis did not detect the presence of O6-methylguanine- DNA methyltransferase promoter methylation.
Still eager to elucidate the origin of this tumor, the patient was referred to the MD Anderson Cancer Center for a new pathology review. The reviewed immunohistochemistry proved positive for CD31, focally positive for factor VIII and S100 and negative for CD34, BCL2, EMA, actin, and desmin [
DISCUSSION
AS may involve the central nervous system (CNS) as metastatic lesions or as primary neoplasms, metastatic origin being the most common. Primary AS of the CNS (PACNS) is a rare condition, accounting for 1–2% of all primary intracranial tumors.[
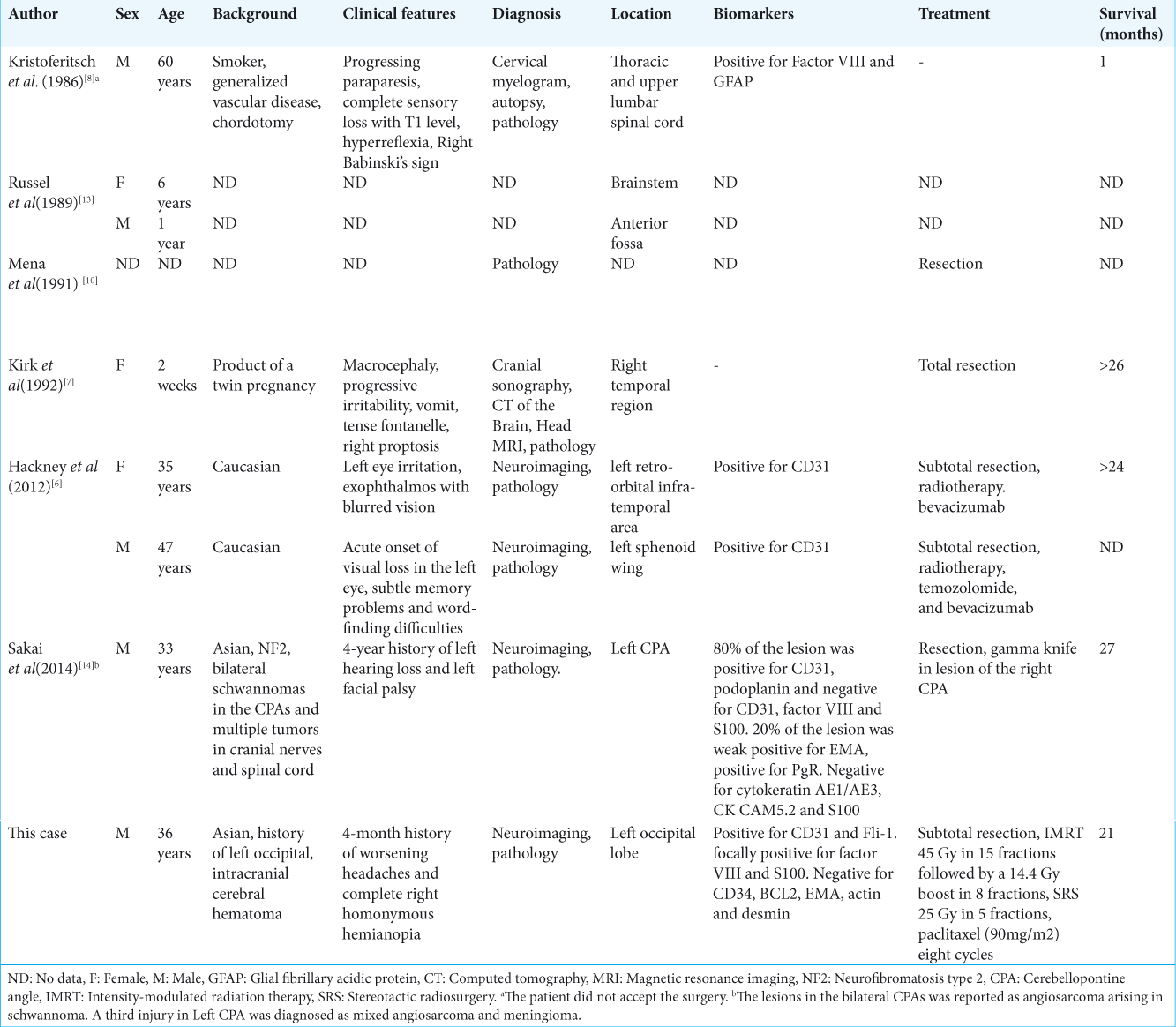
In the past 43 years, 33 cases of PACNS have been reported in the medical literature;[
Because of the heterogeneous nature of the lesions, several immunohistological markers had been used, including CD31, CD34, and factor VIII. The first two are expressed in epithelioid vascular tumors, CD31 being the most accepted. Other described markers are vementin, S100, HMB-45, MIB-1, UEA-1, and GFAP; particularly useful in making differential diagnosis with sarcoma, hemangioblastoma, solitary fibrous tumor, hemangioendothelioma, cavernous angioma, and carcinoma metastases, or epithelious melanoma.[
The low frequency of patients affected by this entity and the great variability of histological subtypes make it unlikely for clinical trials to be conducted; in view of the latter, evidence pointing to optimized therapeutic interventions remains severely precluded. The United Kingdom guidelines for the management of sarcoma recommend surgery with wide resection margins as standard treatment in patients with localized disease. In our patient, gross total resection was problematic due to the aforementioned anatomical constrains. For patients with partial resection, intermediate or high grade tumors, neoadjuvant, or adjuvant radiotherapy is recommended.[
Although treatments for PACNS are not standardized, some studies showed that paclitaxel and bevacizumab are active against AS.[
Studies evaluating the efficacy and safety of chemotherapy for the treatment of AS have been performed in tumors with extracranial localization. For intracranial AS, chemotherapy does not appear to be effective, since most medications do not penetrate the blood–brain barrier.[
The blood–brain barrier is indeed a crucial issue for PACNS, even after a possible increase of the permeability after high- dose radiation.[
CONCLUSION
This case of a meningeal PACNS highlights the importance of considering this entity in the face of a malignant lesion presenting with intracranial hemorrhagic activity. Our observations suggest that the use of paclitaxel provided a modest clinical response in PACNS, highlighting the need to consider a combined approach mainly structured mainly on surgery and radiotherapy. In this context, stereotactic radiosurgery appears to be a promising treatment option. The development of clinical research evaluating the efficacy of systemic agents in the context of PACNS is necessary, taking into account the limitations of drug distribution in the microenvironment of the CNS.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Agulnik M, Yarber JL, Okuno SH, von Mehren M, Jovanovic BD, Brockstein BE. An open-label, multicenter, phase II study of bevacizumab for the treatment of angiosarcoma and epithelioid hemangioendotheliomas. Ann Oncol. 2013. 24: 257-63
2. España P, Chang P, Wiernik PH. Increased incidence of brain metastases in sarcoma patients. Cancer. 1980. 45: 377-80
3. Fellner S, Bauer B, Miller DS, Schaffrik M, Fankhänel M, Spruss T. Transport of paclitaxel (taxol) across the blood-brain barrier in vitro and in vivo. J Clin Invest. 2002. 110: 1309-18
4. Flannery T, Kano H, Niranjan A, Monaco EA, Flickinger JC, Kofler J. Gamma knife radiosurgery as a therapeutic strategy for intracranial sarcomatous metastases. Int J Radiat Oncol Biol Phys. 2010. 76: 513-9
5. Grimer R, Judson I, Peake D, Seddon B. Guidelines for the management of soft tissue sarcomas. Sarcoma. 2010. 2010: 1-15
6. Hackney JR, Palmer CA, Riley KO, Cure JK, Fathallah-Shaykh HM, Nabors LB. Primary central nervous system angiosarcoma: Two case reports. J Med Case Rep. 2012. 6: 251-
7. Kirk IR, Dominguez R, Castillo M. Congenital primary cerebral angiosarcoma: CT, US, and MR findings. Pediatr Radiol. 1992. 22: 134-5
8. Kristoferitsch W, Jellinger K. Multifocal spinal angiosarcoma after chordotomy. Acta Neurochir (Wien). 1986. 79: 145-53
9. LaCorte E, Acerbi F, Schiariti M, Broggi M, Maderna E, Pollo B. Primary central nervous system angiosarcoma: A case report and literature review. Neuropathology. 2015. 35: 184-91
10. Mena H, Ribas JL, Enzinger FM, Parisi JE. Primary angiosarcoma of the central nervous system. J Neurosurg. 1991. 75: 73-6
11. Penel N, Bui BN, Bay JO, Cupissol D, Ray-Coquard I, Piperno-Neumann S. Phase II trial of weekly paclitaxel for unresectable angiosarcoma: The ANGIOTAX study. J Clin Oncol. 2008. 26: 5269-74
12. Ray-Coquard IL, Domont J, Tresch-Bruneel E, Bompas E, Cassier PA, Mir O. Paclitaxel given once per week with or without bevacizumab in patients with advanced angiosarcoma: A randomized phase II trial. J Clin Oncol. 2015. 33: 2797-802
13. Russell DS, Rubinstein LJ.editors. Pathology of Tumors of the Nervous System. London: Arnold; 1989. p. 776-7
14. Sakai Y, Hirose T, Tomono A, Kawakami F, Nakai T, Ohbayashi C. Angiosarcoma arising in schwannoma of cerebellopontine angle and later associating with meningioma in a patient with neurofibromatosis Type 2. Brain Tumor Pathol. 2014. 31: 293-8
15. Zakaria Z, Tambirajoo K, Sattar MT, Farrell MA. Rapid clinical course of multiple metastatic cerebral angiosarcoma. Turk Neurosurg. 2015. 25: 643-8