- Department of Neurosurgery, Toyama University Hospital, Toyama, Japan.
Correspondence Address:
Daina Kashiwazaki, Department of Neurosurgery, Toyama University Hospital, Toyama, Japan.
DOI:10.25259/SNI_417_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Takaki Marutani, Daina Kashiwazaki, Shusuke Yamamoto, Naoki Akioka, Emiko Hori, Satoshi Kuroda. Therapeutic strategy of severe circular calcified carotid plaque with hemodynamic impairment: A patient treated by carotid endarterectomy following balloon angioplasty to prevent hyperperfusion. 12-Aug-2022;13:360
How to cite this URL: Takaki Marutani, Daina Kashiwazaki, Shusuke Yamamoto, Naoki Akioka, Emiko Hori, Satoshi Kuroda. Therapeutic strategy of severe circular calcified carotid plaque with hemodynamic impairment: A patient treated by carotid endarterectomy following balloon angioplasty to prevent hyperperfusion. 12-Aug-2022;13:360. Available from: https://surgicalneurologyint.com/surgicalint-articles/11785/
Abstract
Background: Cerebral hyperperfusion syndrome (HPS) is a serious complication. Recently, staged angioplasty has been reported as an effective strategy to avoid HPS. Severe calcification has been reported as contraindication of carotid artery stenting (CAS). In these cases, carotid endarterectomy (CEA) might be an alternative second stage treatment. We present a case of severe circular calcified plaque with hemodynamic impairments, treated with CEA following percutaneous transluminal angioplasty (PTA) to prevent HPS.
Case Description: A 77-year-old woman presented with severe stenosis at the proximal left internal carotid artery. A CT scan of the neck demonstrated circular calcification. 123I-iodoamphetamine single-photon emission computed tomography (123I-IMP SPECT) showed reductions in cerebral blood flow (CBF) and cerebral vascular reserve in the left hemisphere. Staged therapy was subsequently performed as this patient had a high risk of HPS after conventional CAS or CEA. In the first stage, PTA was performed under local anesthesia. Two days after the procedure, 123I-IMP SPECT revealed improvements in CBF. There were no neurological morbidities. CEA was then performed under general anesthesia 7 days later, for the second stage. We found a calcified plaque with a large thrombus at its proximal end. A hematoxylin-eosin stain of the thrombus showed mostly intact and partially lytic blood cells. Postoperative 123I-IMP SPECT revealed CBF was improved, with no hyperperfusion immediately and 2 days after CEA. The patient was discharged with no neurological deficits.
Conclusion: CEA following PTA for severe circular calcified plaque can be an alternative treatment strategy to prevent HPS. A disadvantage is the formation of thrombi. Early CEA should be considered if thrombus formation is suspected.
Keywords: Calcified plaque, Carotid endarterectomy, Hyperperfusion, Staged angioplasty
INTRODUCTION
Cerebral hyperperfusion syndrome (HPS) is a serious complication of carotid endarterectomy (CEA) or carotid artery stenting (CAS) and can lead to mortality or severe morbidities such as headache, psychological symptoms, focal neurologic deficits, epilepsy, and intracerebral hemorrhage.[
CASE PRESENTATION
A 77-year-old woman presented to our hospital with a history of hypertension, hyperlipidemia, and diabetic mellitus. She also had occlusion of the left central retinal artery due to ipsilateral carotid stenosis 3 years prior, after which no further ischemic events had occurred. Neck MRA demonstrated severe stenosis of the proximal left internal carotid artery (ICA), and the external carotid artery was not detected [
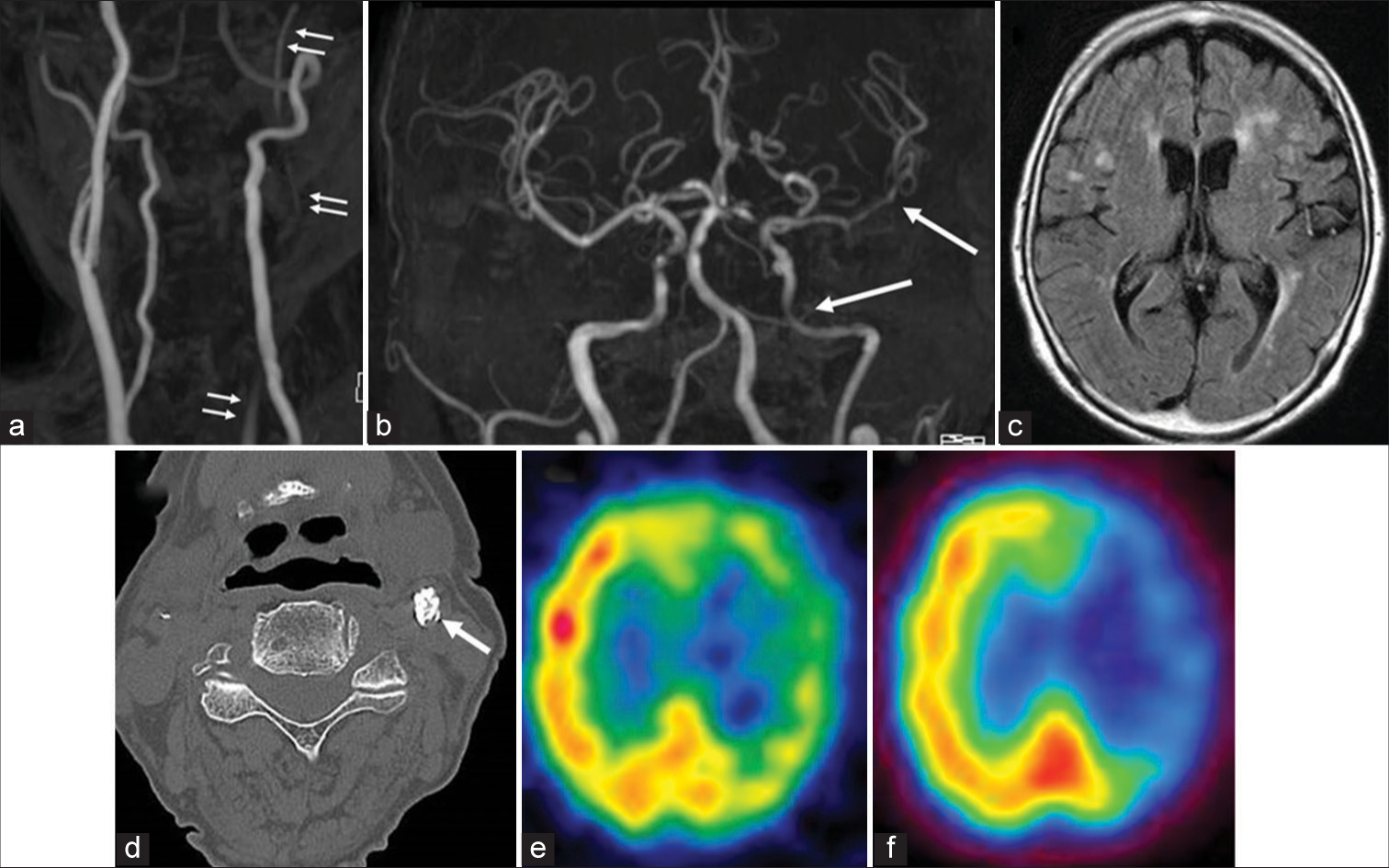
Figure 1:
(a) Neck MRA revealing severe stenosis of the proximal left internal carotid artery (ICA) (arrow), and undetected external carotid artery. (b) Intracranial MRA displaying weak flow signal intensity of the left ICA and left middle cerebral artery (MCA) (arrow). (c) MRI FLAIR showing hyperintense vessel sign in the left MCA. (d) Neck noncontrast CT demonstrating severe circular calcification (arrow). (e and f) 123I-iodoamphetamine single-photon emission computed tomography showing reductions in cerebral blood flow (CBF) and (f) CBF to acetazolamide in the left hemisphere.
Staged therapy was subsequently performed, as this patient had a high risk of HPS following conventional CAS or CEA. Dual antiplatelet therapy (DAPT) (aspirin 100 mg and clopidogrel 75 mg) was administrated 2 weeks before PTA. The procedure was performed under local anesthesia. After intravenous administration of 5000 U heparin, the lesion was crossed with a 0.014 inch guide wire, and submaximal angioplasty was performed using a 2.5 mm × 12 mm noncompliant Gateway® balloon (Stryker Neurovascular, Kalamazoo, Michigan). The stenotic lesion was sufficiently expanded immediately; however, elastic recoil was observed ten minutes later. PTA was then performed using a 3.0 mm × 12 mm Gateway® balloon. After expanding the stenotic lesion, we observed that flow delay was improved [
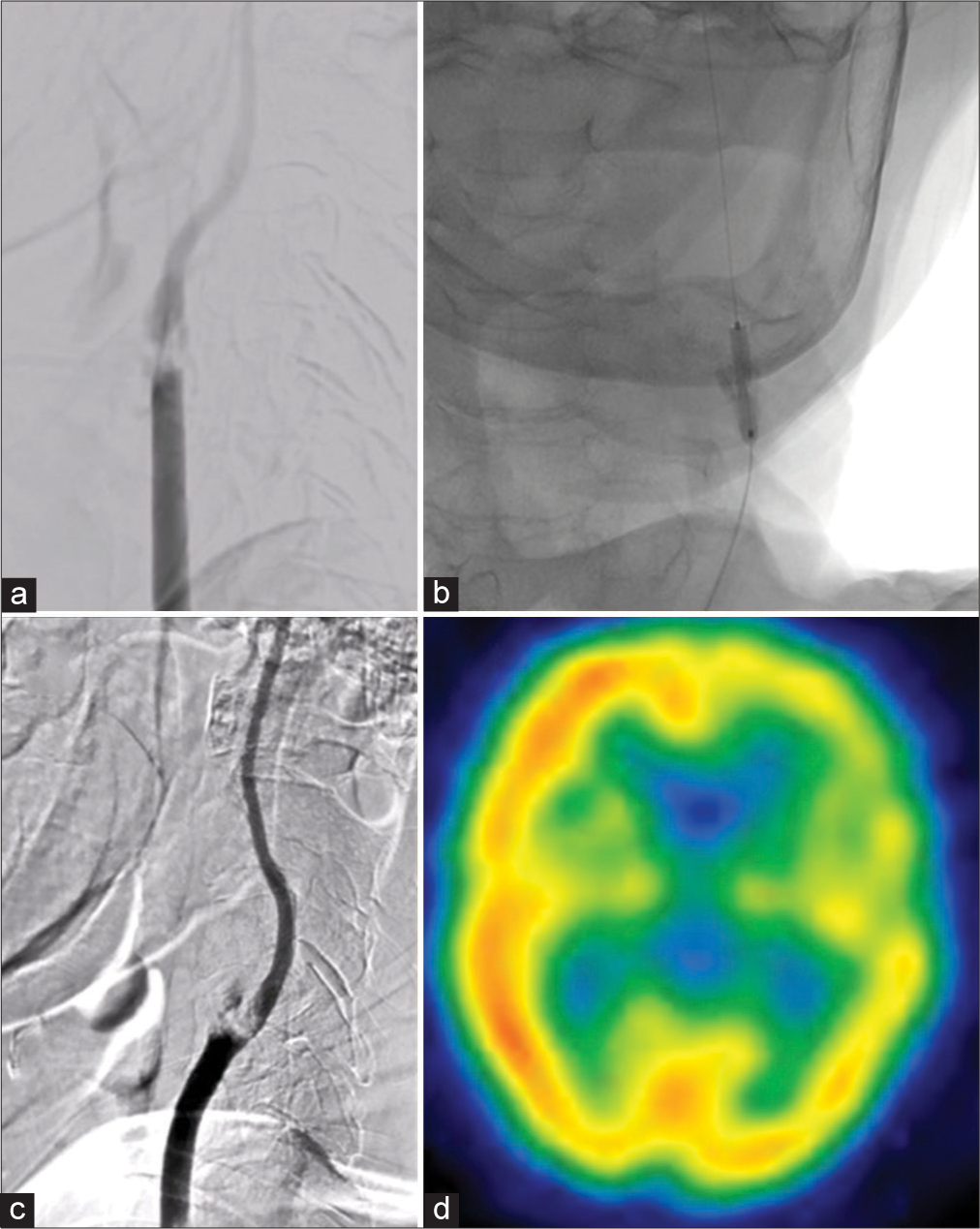
Figure 2:
(a) Left common carotid artery angiography before first-stage percutaneous transluminal angioplasty (PTA) showing slow flow in the internal carotid artery (ICA), and occlusion of the left external carotid artery. (b) PTA being performed. (c) Improved flow within the ICA. (d) Improvements in the left hemisphere cerebral blood flow through 123I-iodoamphetamine single-photon emission computed tomography, but with impairments still detected.
In the second stage, CEA was conducted under general anesthesia 7 days after the first stage, using real-time near-infrared spectroscopy (NIRS) monitoring. The carotid sheath was dissected, and the arteriotomy was completed. Furui’s double balloon shunt system was used to maintain blood flow from the common carotid artery to the ICA. When the ICA was clamped, left cerebral hemisphere NIRS dropped by 10%. However, once reperfusion to ICA was delivered through the shunt system, NIRS returned to baseline. Further, we found a calcified plaque with a large thrombus at its proximal end [
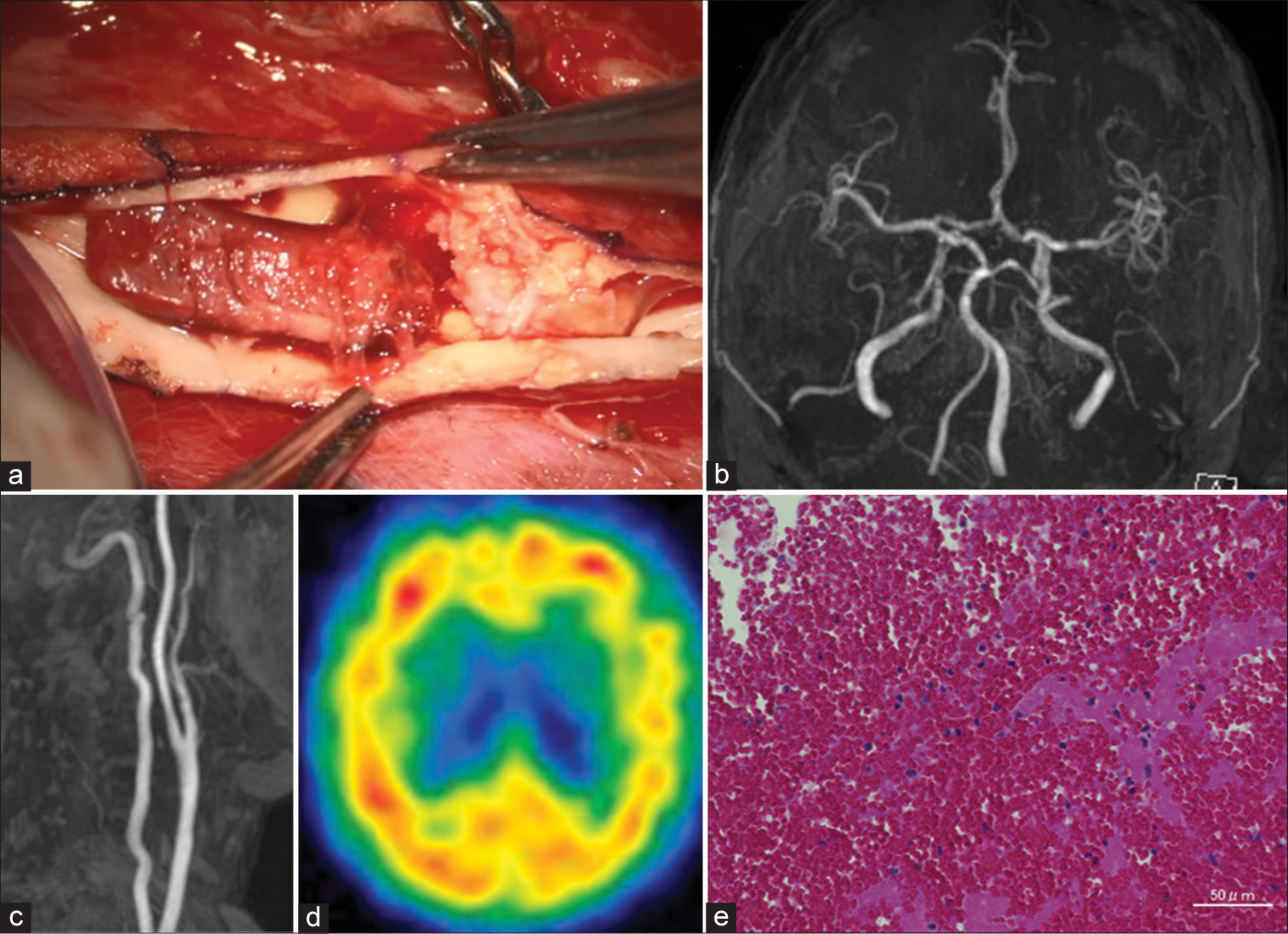
Figure 3:
(a) Operative view of carotid endarterectomy (CEA) showing calcified plaque and thrombus formation proximal to the plaque. (b and c) Improvements in intracranial and neck MRA following CEA. (d) 123I-iodoamphetamine single-photon emission computed tomography 2 days after CEA showing normalized cerebral blood flow. (e) H-E stain of thrombosis revealing mostly intact and partially lytic blood cells, without necrotic tissue.
Histopathology
The extracted thrombus was found to consist of calcified plaque. A hematoxylin-eosin stain of the thrombus showed mostly intact and partially lytic blood cells, without necrotic tissue [
DISCUSSION
In this report, we present a patient with severe circular calcified carotid plaque accompanied by hemodynamic impairments, which were treated with CEA following PTA. This could be an alternative strategy to treat carotid stenosis and prevent HPS. In addition, in this case, thrombus formation occurred following first stage PTA. We have, further, discussed the role and the advantages and disadvantages of staged angioplasty for severe calcified plaques.
Staged revascularization
Conventional staged angioplasty is a two-stage treatment, composing of PTA in the first stage and CAS in the second stage.[
Although the risk and benefit should be consider in each patient, we believe staged revascularization with CEA following PTA could bring more benefit rather than conventional CEA or CAS. Uchida et al. reported 43 patients treated with staged angioplasty (CAS following PTA).[
In our case, first PTA was performed without distal protection, because lumen was too narrow and filter device or protection balloon was not reach to distal of lesion. It is safer using protection device to prevent distal embolism, if filter device or protection balloon can be navigated. In some cases, PTA for circumferential calcified lesions may failed to dilate the lesion. In these cases, CEA should be performed, followed by postoperative hypotensive therapy. If HPS is observed, general anesthesia should be used to prevent the following HPS.
Hyperperfusion and the role of first stage PTA
Impaired cerebral autoregulation is a known mechanism of hyperperfusion. With concomitant single-stage CEA/ CAS, CBF over the under-perfused area would drastically increase. Normal cerebral autoregulation constricts the cerebral artery in response to a sudden increase in blood flow, to maintain normal cerebral perfusion. Patients with impaired hemodynamic function additionally cannot maintain a stable CBF through vasoconstriction in response to a sudden increase in cerebral perfusion pressure after revascularization.[
CONCLUSION
CEA following PTA for severe circular calcified plaque can be an alternative treatment strategy to prevent HPS However, the optimal interval period between the initial PTA and CEA is unclear. A disadvantage of this staged procedure is the formation of thrombi. Early CEA should be considered if thrombus formation is suspected.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Chang CK, Huded CP, Nolan BW, Powell RJ. Prevalence and clinical significance of stent fracture and deformation following carotid artery stenting. J Vasc Surg. 2011. 54: 685-90
2. Clark DJ, Lessio S, O’Donoghue M, Schainfeld R, Rosenfield K. Safety and utility of intravascular ultrasound-guided carotid artery stenting. Catheter Cardiovasc Interv. 2004. 63: 355-62
3. Derdeyn CP, Videen TO, Yundt KD, Fritsch SM, Carpenter DA, Grubb RL. Variability of cerebral blood volume and oxygen extraction: Stages of cerebral haemodynamic impairment revisited. Brain. 2002. 125: 595-607
4. Egashira Y, Yoshimura S, Yamada K, Enomoto Y, Asano T, Iwama T. Stepwise revascularization by carotid endarterectomy after balloon angioplasty for symptomatic severe carotid artery stenosis. Ann Vasc Surg. 2012. 26: 13.e9-13
5. Fan X, Zuo Z, Lin T, Lai Z, You H, Qu J. Arterial transit artifacts on arterial spin labeling MRI can predict cerebral hyperperfusion after carotid endarterectomy: An initial study. Eur Radiol. 2022. p.
6. Hayakawa M, Sugiu K, Yoshimura S, Hishikawa T, Yamagami H, Fukuda-Doi M. Effectiveness of staged angioplasty for avoidance of cerebral hyperperfusion syndrome after carotid revascularization. J Neurosurg. 2019. 132: 1-11
7. Ho DS, Wang Y, Chui M, Ho SL, Cheung RT. Epileptic seizures attributed to cerebral hyperperfusion after percutaneous transluminal angioplasty and stenting of the internal carotid artery. Cerebrovasc Dis. 2000. 10: 374-9
8. Hussain MA, Alali AS, Mamdani M, Tu JV, Saposnik G, Salata K. Risk of intracranial hemorrhage after carotid artery stenting versus endarterectomy: A population-based study. J Neurosurg. 2018. 129: 1522-9
9. Katano H, Yamada K. Analysis of calcium in carotid plaques with Agatston scores for appropriate selection of surgical intervention. Stroke. 2007. 38: 3040-4
10. Li X, Kramer MC, Van Der Loos CM, Ploegmakers HJ, De Boer OJ, Koch KT. Early onset of endothelial cell proliferation in coronary thrombi of patients with an acute myocardial infarction: Implications for plaque healing. J Thromb Haemost. 2012. 10: 466-73
11. Maas MB, Kwolek CJ, Hirsch JA, Jaff MR, Rordorf GA. Clinical risk predictors for cerebral hyperperfusion syndrome after carotid endarterectomy. J Neurol Neurosurg Psychiatry. 2013. 84: 569-72
12. Reimers B, von Birgelen C, van der Giessen WJ, Serruys PW. A word of caution on optimizing stent deployment in calcified lesions: Acute coronary rupture with cardiac tamponade. Am Heart J. 1996. 131: 192-4
13. Rittersma SZ, van der Wal AC, Koch KT, Piek JJ, Henriques JP, Mulder KJ. Plaque instability frequently occurs days or weeks before occlusive coronary thrombosis: A pathological thrombectomy study in primary percutaneous coronary intervention. Circulation. 2005. 111: 1160-5
14. Takahashi M, Takamoto T, Aizawa T, Shimada H. Severity of coronary artery calcification detected by electron beam computed tomography is related to the risk of restenosis after percutaneous transluminal coronary angioplasty. Intern Med. 1997. 36: 255-62
15. Uchida K, Yoshimura S, Shirakawa M, Shindo S, Egashira Y, Iwama T. Experience of staged angioplasty to avoid hyperperfusion syndrome for carotid artery stenosis. Neurol Med Chir (Tokyo). 2015. 55: 824-9
16. Yoshimura S, Kitajima H, Enomoto Y, Yamada K, Iwama T. Staged angioplasty for carotid artery stenosis to prevent postoperative hyperperfusion. Neurosurgery. 2009. 64: s122-8