- Chief of Neurosurgical Spine and Education, Department of Neuro Science, Winthrop University Hospital, Mineola, NY 11501, USA
Correspondence Address:
Nancy E. Epstein
Chief of Neurosurgical Spine and Education, Department of Neuro Science, Winthrop University Hospital, Mineola, NY 11501, USA
DOI:10.4103/2152-7806.156561
Copyright: © 2015 Epstein NE. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.How to cite this article: Epstein NE. Tisseel does not reduce postoperative drainage, length of stay, and transfusion requirements for lumbar laminectomy with noninstrumented fusion versus laminectomy alone. Surg Neurol Int 07-May-2015;6:
How to cite this URL: Epstein NE. Tisseel does not reduce postoperative drainage, length of stay, and transfusion requirements for lumbar laminectomy with noninstrumented fusion versus laminectomy alone. Surg Neurol Int 07-May-2015;6:. Available from: http://surgicalneurologyint.com/surgicalint_articles/tisseel-not-reduce-postoperative-drainage-length-stay/
Abstract
Background:Typically, fibrin sealants (FSs) and fibrin glues (FGs) are used to strengthen dural repairs during spinal surgery. In 2014, Epstein demonstrated that one FS/FG, Tisseel (Baxter International Inc., Westlake Village, CA, USA) equalized the average times to drain removal and length of stay (LOS) for patients with versus without excess bleeding (e.g. who did not receive Tisseel) undergoing multilevel laminectomies with 1-2 level noninstrumented fusions (LamF).[
Methods:Here Tisseel was utilized to promote hemostasis for two populations; 39 patients undergoing average 4.4 level lumbar laminectomies with average 1.3 level noninstrumented fusions (LamF), and 48 patients undergoing average 4.0 level laminectomies alone (Lam). We compared the average operative time, estimated blood loss (EBL), postoperative drainage, LOS, and transfusion requirements for the LamF versus Lam groups.
Results:The average operative times, EBL, postoperative drainage, LOS, and transfusion requirements were all greater for LamF versus Lam patients; operative times (4.1 vs. 3.0 h), average EBL (192.3 vs. 147.9 cc), drainage (e.g. day 1; 199.6 vs. 167.4 cc; day 2; 172.9 vs. 63.9 cc), average LOS (4.6 vs. 2.5 days), and transfusion requirements (11 LamF patients; 18 Units [U] RBC versus 2 Lam patients; 3 U RBC).
Conclusions:Utilizing Tisseel to facilitate hemostasis in LamF versus Lam still resulted in greater operative times, EBL, postoperative average drainage, LOS, and transfusion requirements for patients undergoing the noninstrumented fusions. Although Tisseel decreases back bleeding within the spinal canal, it does not reduce blood loss from LamF decorticated transverse processes.
Keywords: Fibrin sealant, hemostasis, laminectomy alone, length of stay, lumbar surgery, multilevel laminectomy, noninstrumented fusion, stenosis, tisseel
INTRODUCTION
Typically, fibrin sealants (FSs) and fibrin glues (FGs) have been utilized to strengthen repairs of deliberate (e.g. intradural tumors) or traumatic cerebrospinal fluid (CSF) fistulas occurring during spinal surgery. In 2014, Epstein documented that one FS, Tiseeel (Baxter International Inc., Westlake Village, CA, USA), equalized the average times to drain removal and length of stay (LOS) for patients undergoing multilevel laminectomies and 1-2 level noninstrumented fusions (LamF) with versus without increased bleeding (the latter did not receive Tisseel).[
MATERIALS AND METHODS
Thirty-nine patients undergoing multilevel laminectomies/noninstrumented fusions
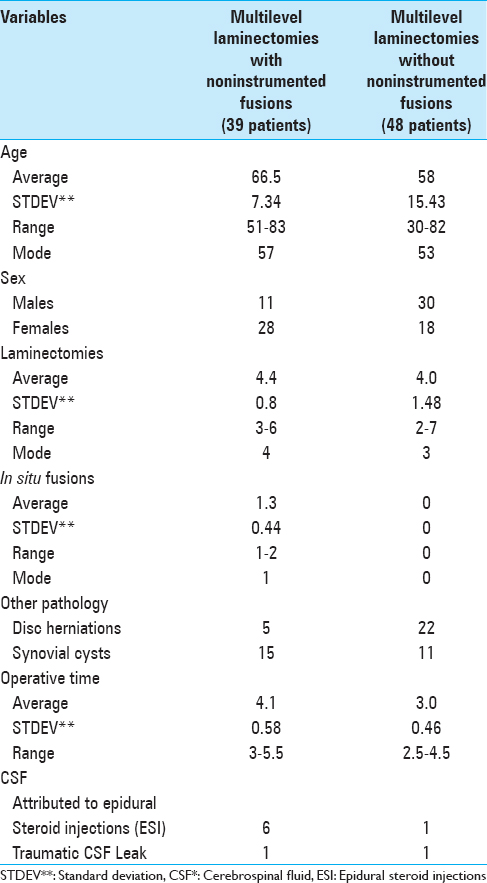
The 39 patients undergoing LamF averaged 66.5 years of age, and included more females than males (28 females, 11 males) [
Forty-eight patients undergoing multilevel laminectomies alone without fusions
The 48 patients undergoing Lam averaged 58 years of age, and included more males than females (30 males 18 females) [
Use of tisseel to facilitate hemostasis
All patients in both series undergoing multilevel laminectomies with noninstrumented fusions (LamF) or with Lam alone received Tisseel intraoperatively to facilitate hemostasis in the spinal canal (e.g. from the epidural venous plexus or laminectomy bone back-bleeding). Additionally, Tisseel was utilized to facilitate treatment of CSF fistulas for LamF versus Lam patients; six LamF and one Lam patient exhibited CSF fistulas attributed to preoperative epidural steroid injections (ESI), while one patient in each group exhibited a traumatic intraoperative CSF fistula [
RESULTS
Greater EBL, postoperative drainage, time to drain removal, and longer length of stay for lamF versus Lam alone patients
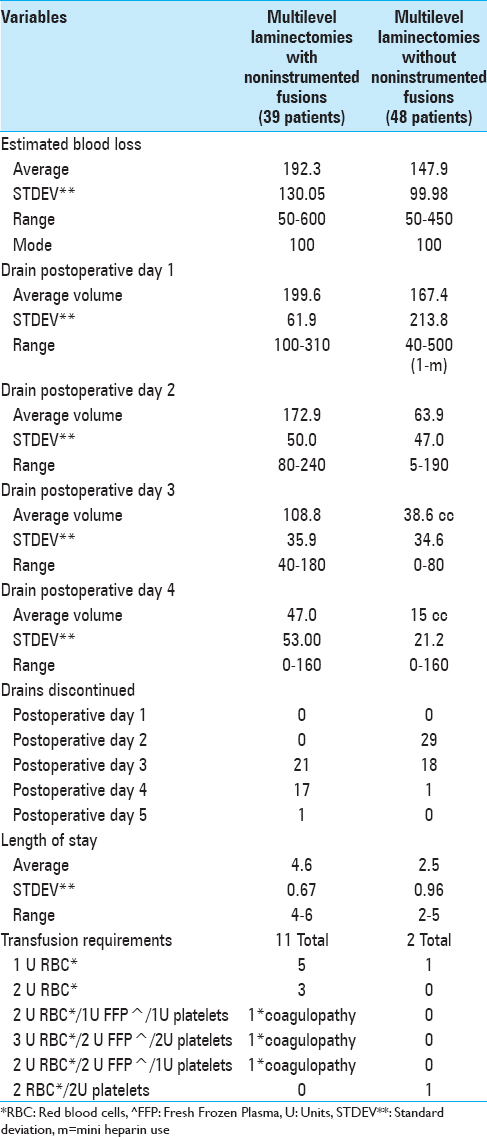
LamF patients exhibited greater EBL, postoperative drainage, longer times to drain removal, and LOS versus Lam alone patients. The intraoperative EBL was greater for LamF versus Lam patients (192.3 vs. 147.9 cc). The average amount of daily postoperative drainage was greater for LamF versus Lam patients particularly on postoperative days 1 and 2 when drains had not yet been removed in any patients; day 1: 199.6 versus 167.4 cc, and day 2; 172.9 versus 63.9 cc [
Greater transfusion requirements for LamF versus Lam alone patients
LamF patients additionally exhibited greater transfusion requirements due to back-bleeding from decorticated transverse processes (TP) occurring during noninstrumented fusions versus Lam alone [
DISCUSSION
Frequency of dural tears
Traumatic dural tears (DT) occur during 1–17% of lumbar spinal surgical procedures, 1% for anterior cervical discectomy/fusion (ACDF), 1.7% for single-level anterior cervical corpectomy/fusion (ACF), and up to 12.5% for multilevel ACF involving resection of ossification of the posterior longitudinal ligament (OPLL).[
Safety/efficacy of “fibrin sealants” and “fibrin glues” to repair DT
Epstein reviewed the relative safety/efficacy of supplementing/strengthening repairs of spinal DT utilizing two “FSs” or two “FG” [
Paralytic complications with duraseal (FS)
Although the Food and Drug Administration (FDA) approved Duraseal (FS) for use in the brain and spine in 2009, Duraseal contributed to two cases of reported paralysis due to spinal injections. In 2009, Mulder et al. noted that DuraSeal, applied in the lumbar spine to facilitate DT repair following a laminotomy/diskectomy, migrated superiorly, became “swollen,” and contributed to a cauda equina syndrome 6 days postoperatively.[
Use of tisseel for hemostasis in cranial and anterior cervical surgery
Injecting Tisseel facilitated hemostasis both intracranially and in the anterior cervical spine. In 2007, Sekhar et al. safely achieved hemostasis by injecting Tisseel into the cranial epidural space (n = 200 patients), anterior cavernous sinus (n = 46 patients), and vertebral venous plexus (n = 20 patients); however, it resulted in venous occlusion/brain stem infarction when injected into the superior petrosal sinus.[
Sandwich versus conventional dural repair techniques utilizing fibrin sealant
Wang et al. compared the efficacy of utilizing the “sandwich” (interlocking suture/FS/FG glue/gelatin sponge/FS/FG glue) versus conventional (interlocking sutures/gelatin sponge) techniques for dural repair following removal of 54 spinal subdural tumors to prevent recurrent postoperative CSF leaks.[
Tisseel's safety/efficacy in reducing spinal surgical site infections
In 2012 and 2013, two authors noted that Tisseel impregnated with antibiotics reduced the risk of spinal infection.[
FS and evicel; successful hemostatic agents in total joint surgery
In total joint surgery in 2009, Thoms and Marwin found that FS reduced the time to achieve intraoperative hemostasis, EBL, and the incidence of infections, while facilitating wound healing, and increasing postoperative range of motion.[
Different FS/FG promote intraoperative lumbar spinal hemostasis
Different FS/FG have been utilized to promote intraoperative lumbar spinal hemostasis.[
Tisseel in LamF patients undergoing noninstrumented postero-lateral fusions does not limit back bleeding from decorticated transverse processes
Despite the use of Tisseel to facilitate hemostasis in the LamF versus Lam groups, noninstrumented fusions with decorticated TP for uniquely contributed to greater operative times, intraoperative EBL, postoperative drainage, LOS, and transfusion requirements. Although LamF versus Lam patients underwent nearly comparable multilevel laminectomies (4.4 vs. 4.0 levels), the average 1.3 level noninstrumented fusions were responsible for these differences. LamF patients exhibited longer operative times (e.g. 1.1 h more), higher average EBL (44.4 cc), greater average daily drainage (day 1: 32.3 cc, day 2: 109 cc), longer times until drain removal (e.g. LamF days 3–4 [38 of 39 patients] vs. Lam days 2–3 [47 of 48 patients]), greater transfusion requirements (11 LamF patients requiring 18 U RBC vs. 2 Lam patients requiring 3U RBC), and longer LOS (average 4.6 vs. 2.5 days).
CONCLUSION
Tisseel, applied in the lumbar spinal canal locally limits drainage from epidural veins and largely stops back bleeding from the exposed edges of laminectomy defects. It cannot, however, limit back bleeding from TP decorticated during the performance of noninstrumented postero-lateral fusions. The intent of this study was to document for LamF versus Lam patients that the noninstrumented fusions contributed to the longer operative times, greater average EBL, higher volume of postoperative drainage, longer LOS, and higher transfusion requirements. These data may prove useful when deciding whether to perform LamF versus Lam alone especially in older patients with greater comorbidities who may not tolerate the greater risks enumerated above associated with the noninstrumented fusions.
References
1. Bou Monsef J, Buckup J, Waldstein W, Cornell C, Boettner F. Fibrin sealants or cell saver eliminate the need for autologous blood donation in anemic patients undergoing primary total knee arthroplasty. Arch Orthop Trauma Surg. 2014. 134: 53-8
2. Cammisa FP, Girardi FP, Sangani PK, Parvataneni HK, Cadag S, Sandhu HS. Incidental durotomy in spine surgery. Spine (Phila Pa 1976). 2000. 25: 2663-7
3. Cashman JD, Jackson JK, Mugabe C, Gilchrist S, Ball K, Tredwell S. The use of tissue sealants to deliver antibiotics to an orthopaedic surgical site with a titanium implant. J Orthop Sci. 2013. 18: 165-74
4. Dafford EE, Anderson PA. Comparison of dural repair techniques. Spine J. 2013. pii: S1529-9430
5. Epstein NE. Dural repair with four spinal sealants: Focused review of the manufacturers’ inserts and the current literature. Spine J. 2010. 10: 1065-8
6. Epstein NE. Tisseel utilized as a hemostatic in spine surgery impacts time to drain removal and length of stay. Surg Neurol Int. 2014. 5: S354-61
7. Epstein NE. Commentary: Unnecessary preoperative epidural steroid injections lead to cerebrospinal fluid leaks confirmed during spinal stenosis surgery. Surg Neurol Int. 2014. 5: S325-8
8. Epstein NE. Hemostasis and other benefits of fibrin sealants/glues in spine surgery beyond cerebrospinal fluid leak repairs. Surg Neurol Int. 2014. 5: S304-14
9. Hannalah D, Lee J, Khan M, Donaldson WF, Kang JD. Cerebrospinal fluid leaks following cervical spine surgery. J Bone Joint Surg Am. 2008. 90: 1101-5
10. Jankowitz BT, Atteberry DS, Gerszten PC, Karausky P, Cheng BC, Faught R. Effect of fibrin glue on the prevention of persistent cerebral spinal fluid leakage after incidental durotomy during lumbar spinal surgery. Eur Spine J. 2009. 18: 1169-74
11. Miscusi M, Polli FM, Forcato S, Coman MA, Ricciardi L, Ramieri A. The use of surgical sealants in the repair of dural tears during non-instrumented spinal surgery. Eur Spine J. 2014. 23: 1761-6
12. Mulder M, Crosier J, Dunn R. Cauda equina compression by hydrogel dural sealant after a laminotomy and discectomy: Case report. Spine (Phila Pa 1976). 2009. 34: E144-8
13. Sekhar LN, Natarajan SK, Manning T, Bhagawati D. The use of fibrin glue to stop venous bleeding in the epidural space, vertebral venous plexus, and anterior cavernous sinus: Technical note. Neurosurgery. 2007. 61: E51-
14. Thavarajah D, De Lacy P, Hussain R, Redfern RM. Postoperative cervical cord compression induced by hydrogel (DuraSeal): A possible complication. Spine (Phila Pa 1976). 2010. 35: E25-6
15. Thoms RJ, Marwin SE. The role of fibrin sealants in orthopaedic surgery. J Am Acad Orthop Surg. 2009. 17: 727-36
16. Tofuku K, Koga H, Yanase M, Komiya S. The use of antibiotic-impregnated fibrin sealant for the prevention of surgical site infection associated with spinal instrumentation. Eur Spine J. 2012. 21: 2027-33
17. Wang HR, Cao SS, Jiang YQ, Li JN, Li XL, Fu YG. A comparison between “sandwich” and conventional methods of repairing spinal dura rupture. Orthop Surg. 2012. 4: 233-40
18. Wu J, Jin Y, Zhang J, Shao H, Yang D, Chen J. Hemostatic Techniques Following Multilevel PosteriorLumbar Spine Surgery: A Randomized Control Trial. J Spinal Disord Tech. 2014. 27: 442-6
19. Yeom JS, Buchowski JM, Shen HX, Liu G, Bunmaprasert T, Riew KD. Effect of fibrin sealant on drain output and duration of hospitalization after multilevel anterior cervical fusion: A retrospective matched pair analysis. Spine (Phila Pa 1976). 2008. 33: E543-7