- Department of Neurosurgery, Faculty of Medicine Universitas Airlangga – Dr. Soetomo General Academic Hospital, Surabaya, East Java, Indonesia.
Correspondence Address:
Joni Wahyuhadi, Department of Neurosurgery, Faculty of Medicine Universitas Airlangga – Dr. Soetomo General Academic Hospital, Surabaya, East Java, Indonesia.
DOI:10.25259/SNI_19_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Resi Prastikarunia, Joni Wahyuhadi, Rahadian Indarto Susilo, Irwan Barlian Immadoel Haq. Tranexamic acid to reduce operative blood loss in brain tumor surgery: A meta-analysis. 12-Jul-2021;12:345
How to cite this URL: Resi Prastikarunia, Joni Wahyuhadi, Rahadian Indarto Susilo, Irwan Barlian Immadoel Haq. Tranexamic acid to reduce operative blood loss in brain tumor surgery: A meta-analysis. 12-Jul-2021;12:345. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=10963
Abstract
Background: Major blood loss during neurosurgery may result in a variety of complications, such as potentially fatal hemodynamic instability. Brain tumor and skull base surgery is among the high bleeding risk procedures. Tranexamic acid (TXA) has been found to reduce bleeding events in various fields of medicine.
Methods: We searched for all randomized controlled trials published in English or Bahasa which compared the use of TXA with placebo in brain tumor surgery. The studies should include adult patients with intracranial tumor who received TXA before skin incision. The primary and secondary outcomes are intraoperative blood loss and the need of transfusion.
Results:
Conclusion: TXA reduced the volume of blood loss but did not reduce the need of blood transfusion.
Keywords: Brain tumor, Intraoperative bleeding, Tranexamic acid, Transfusion
INTRODUCTION
Major blood loss during neurosurgical procedures will complicate the treatment and reduce tissue perfusion to vital brain tissue.[
Tranexamic acid (TXA) is a synthetic derivative of the amino acid lysine that acts as antifibrinolytic. TXA has been used in various setting to reduce blood loss.[
MATERIALS AND METHODS
Types of studies
We searched for all randomized controlled trials (RCTs), prospective, and retrospective studies published in English or Bahasa which compared the use of TXA with other agents or placebo.
Types of participants
Adult (≥18 years old) patients of either gender diagnosed with intracranial tumor who underwent craniotomy and tumor resection procedure.
Types of interventions
Studies with intravenous administration of tranexamic acid at any dose, by bolus and/or by intravenous drip, will be included. The comparison could be placebo or other antifibrinolytic agents.
Types of outcome measure
Primary outcome
Mean blood loss
Secondary outcome
The need of PRC and/or whole blood transfusion The need of colloid administration as volume expander
Search methods for identification of studies
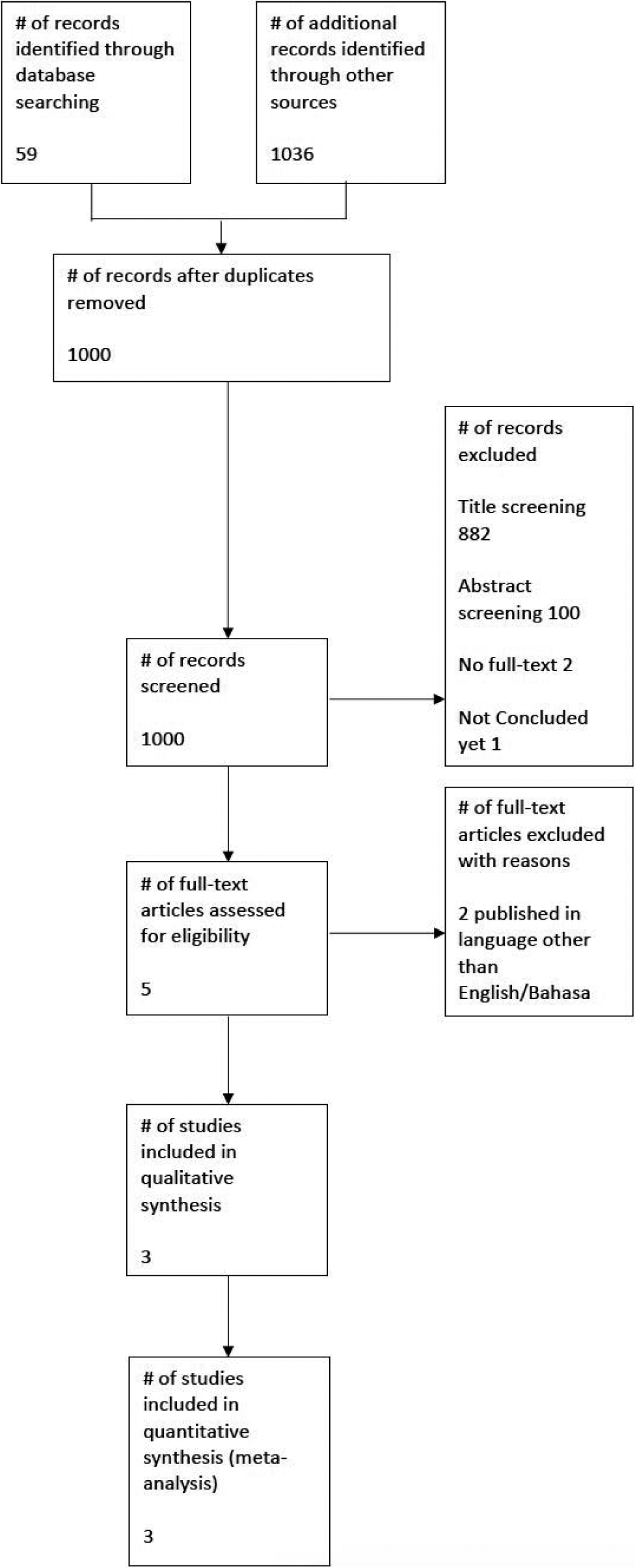
The sampling technique in this study was using online literature search results filtering based on the flow of Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) according to the PICO that has been determined. We searched PubMed, Cochrane Central Register of Controlled Trials (CENTRAL), and ProQuest. Search was limited to papers published between 2010 and 2020. Our search strategy is shown in [
We also searched Google Scholar using any of the possible combination mentioned above.
Data collection and analysis
Selection of studies
The search results were first excluded based on the relevancy of the titles and then on the relevancy of the abstracts. Non-English/non-Bahasa publications were automatically excluded. Full-text articles were then assessed by all authors (JW, RP, IBIH, and RIS) for potentially eligible RCTs. The reasons of exclusion were noted and reported.
Data extraction and management
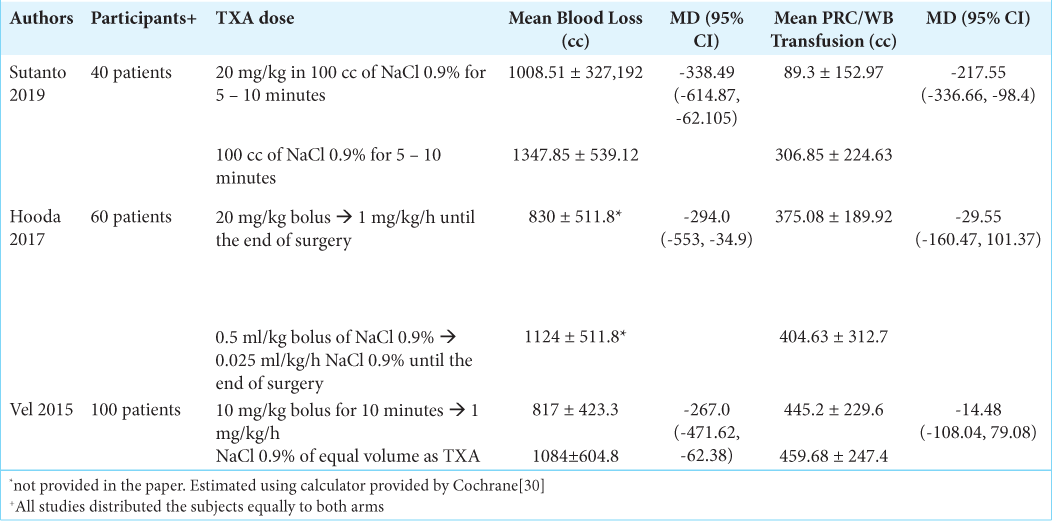
Demographic data about age, sex, diagnosis, TXA dose and administration, blood loss, and transfusion requirement were collected and presented in [
Assessment of risk of bias in included studies
Risk of bias was assessed by all four authors (JW, RP, IBIH, and RIS). Should conclusion be unmet, a third party from neurosurgery department would be asked to give his/ her opinion. Assessed biases are those mentioned in the Cochrane Collaboration Tool for Assessing Risk of Bias in Randomized Trials published in 2011.[
Measures of treatment effect
We undertook statistical analysis using the statistical software, Review Manager 5.4, of the Cochrane Collaboration. We used risk ratios to measure treatment effect for proportions (dichotomous outcomes) among primary and secondary outcomes. Random effect model will be used should evidence of significant heterogeneity is present. A statistically significant difference between intervention and control groups was assumed if the 95% CI did not include the value of no differential effect.
Assessment of heterogeneity
Heterogeneity is addressed by the I2 value on forest plot construction using RevMan 5.4. The statistical model used is switched to random effect should I2 yield the value of ≥50% as the studies are deemed heterogeneous.[
RESULTS
Description of studies
Description of studies can be seen on [
Results of the search
The exclusion processes based on the flow of PRISMA are shown in [
Included studies
After careful consideration from each authors, we decided to include three studies.[
Excluded studies
There are four studies which most likely met our criteria and did report the required data for our primary outcome, but were unfortunately ruled out. Two of them were due to unavailability of full text[
Ongoing studies
By the time of our search, we found one study which has not been concluded yet.[
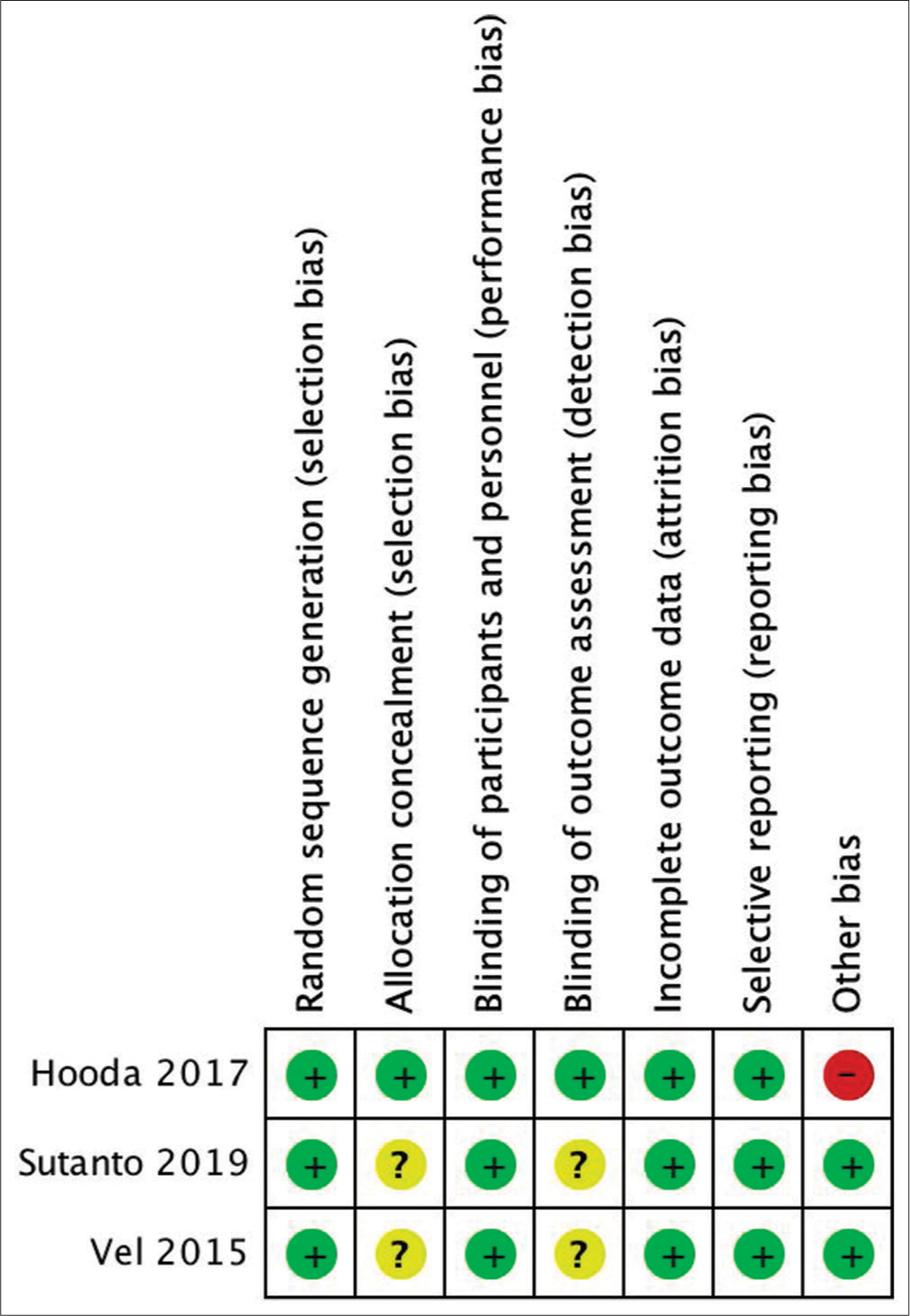
Risk of bias in included studies
Risk of bias of included studies was assessed using the Cochrane Collaboration Tool for Assessing Risk of Bias in Randomized Trials published in 2011.[ Random sequence generation Allocation concealment Blinding of participant and personnel Blinding of outcome and assessment Incomplete outcome data Selective reporting Other bias
The result of the assessment is shown in [
Randomization and allocation concealment
Hooda et al. randomized the study’s subjects using computer-generated randomization chart. The TXA infusion was prepared by an anesthesiologist who was not involved in patient management.[
Blinding
All three studies did blind the neurosurgeons and the anesthesiologists involved in the surgical procedure.[
Incomplete outcome data
All studies’ subjects were included in the result section. No subjects dropped-out of the studies.[
Selective reporting
We found that all outcomes mentioned in the methods section were reported in the studies.[
Other potential sources of bias
Hooda et al. did not report the standard error of the mean blood loss. An attempt to contact the author has not been fruitful. To complete the missing data, we utilized RevMan Calculator tool by Cochrane which is accessible in their website.[
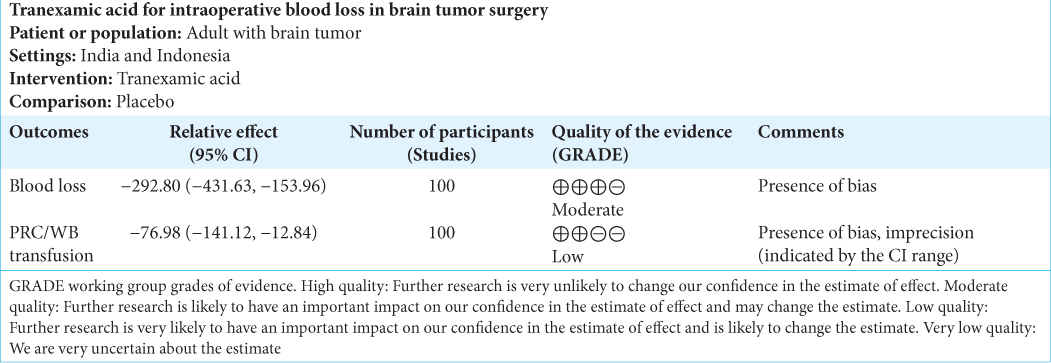
Summary of findings
Summary of findings from each studies and quality of evidence for each outcome can be found in [
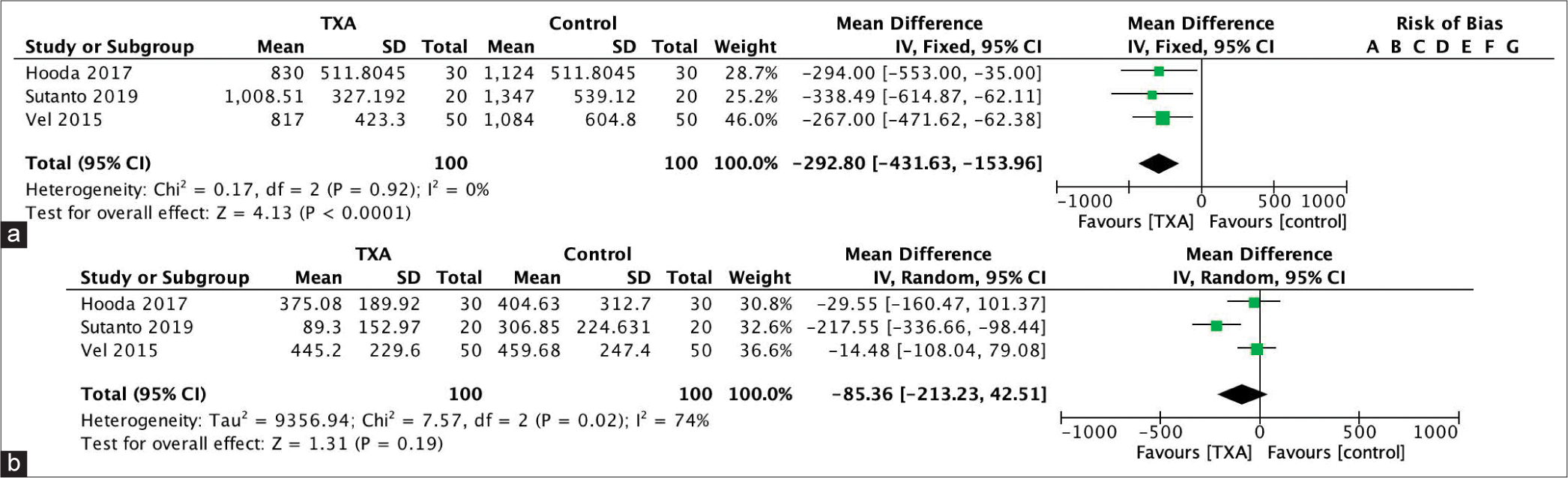
All three included studies reported their results on blood loss and the need of blood transfusion. Sutanto et al. included 40 subjects who were distributed evenly into the study arms. The study compared 20 mg/kg of TXA in 100 cc of NaCl 0.9% with 100 cc of NaCl 0.9%. Either of the infusions was administered for 5–10 min. Baseline PT and aPTT were not different between the two groups. The authors reported that there were patients in the control group who eventually needed fresh frozen plasma (FFP) transfusion (mean 73.50 ± 121.926 cc).[
Hooda et al. had 60 study subjects who were equally distributed into TXA group and placebo group. This study administered TXA continuously until the surgery concluded. The dose was 20 mg/kg bolus continued with continuous drip at a dose of 1 mg/kg/h. The comparison was 0.5 ml/kg bolus of NaCl 0.9% continued with 0.025 ml/kg/h NaCl 0.9% continuous drip. Four patients received FFP transfusion in each group, with administered volume of 600 cc and 900 cc in the TXA and placebo group, respectively. Three patients in the TXA group also received platelet transfusion (total transfused volume 250 cc), while two patients in the placebo group received 306 cc of platelet.[
The differences between studies are (1) timing and ending of TXA administration, (2) type of tumor, (3) tumor size, (4) embolization history, and (5) pregnancy status, as shown in [
Effects of interventions
The effect of intervention is shown in [
DISCUSSION
This meta-analysis sought to find out if TXA can reduce intraoperative blood loss and blood transfusion in brain tumor surgery. We identified five studies but unfortunately needed to exclude two of them. The included studies yield a total of 200 patients. Massive blood loss from surgical procedure has been associated with mortality and morbidity.[
The CRASH-3 trial, the most recent and largest clinical trial about TXA, found that TXA administration in the first 3 h of acute traumatic brain injury (TBI) reduced mortality. However, this trial did not specifically sought intraoperative blood loss or the need of transfusion.[
To this day, the use of TXA in neurosurgery has been limited to TBI or subarachnoid hemorrhage (SAH) cases, primarily due to the fear of thrombotic adverse effects.[
The evidence for use of TXA to treat massive blood loss during intracranial surgery is weak and is even more scarce in terms of brain tumor surgery.[
The administration of TXA has demonstrated positive results in spine procedures. In most studies, the preferred TXA dose ranged from 10 mg/kg to 30 mg/kg immediately on performing an incision, a maintenance dose of 0.5–2 mg/kg/h, followed by a preferable 1-mg/kg/h dose until the end of the surgical procedure. High TXA doses did not necessarily increase rates of thromboembolism or convulsions in the case of ASA I and ASA II patients, with no risk factors for thromboembolism or significant renal changes.[
The trials were pooled in a Cochrane study, which concluded that TXA may reduce mortality in TBI patients, but the standard of evidence is poor and there is significant uncertainty.[
The above trials were pooled in a Cochrane study, which concluded that TXA may reduce mortality in TBI patients, but the standard of evidence is poor and there is significant uncertainty.
Headache, fatigue, vomiting, diarrhea, dyspepsia, dysmenorrhea, dizziness, back pain, numbness, phosphenes, and anemia are some of the side effects of TXA when used for an extended period of time.[
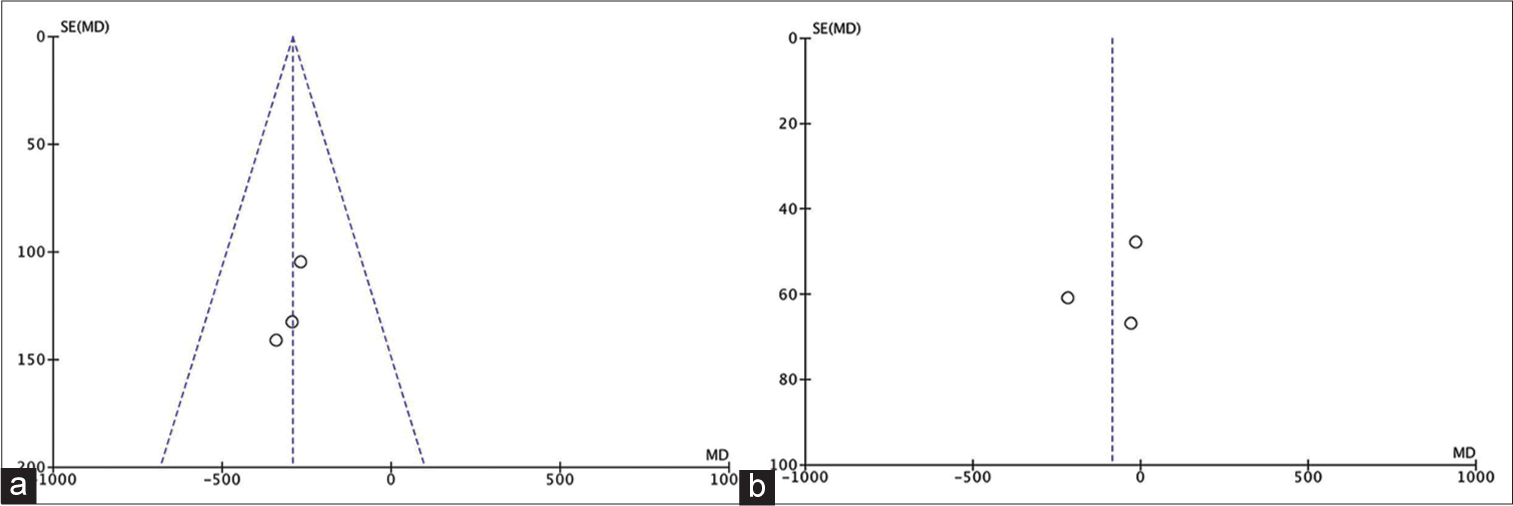
Our meta-analysis revealed that TXA reduced intraoperative blood loss at a mean of 292.80 cc (95% CI, −431.63, −153.96). Despite consisting only of 100 subjects and a relatively few included studies, these studies were considered homogenous. The need of transfusion, however, did not seem to be affected by TXA. The pooled mean difference of blood transfusion was −85.36 (95% CI, −213.23 – [42.51]). The range of CI indicated that the TXA group did not always have less blood transfusion. It is also important to recognize the considerable heterogeneity among studies in regard to transfusion volume.
Overall completeness and applicability of evidence
The overall methodological quality of these studies is considered good. There was, however, a considerable heterogeneity in respect to the secondary outcome. One study did not provide the standard error of both mean blood loss and mean blood transfusion. The inclusion criteria between studies were quite similar as well.
Quality of the evidence
We deem that the conclusion for both our primary and secondary outcomes belongs to moderate and low-quality evidence, respectively, mainly due to the presence of at least one type of bias in all of the studies and the inconsistency with regard to transfusion volume. The studies were also heterogeneous in terms of transfusion volume.
Agreements and disagreements with previous meta-analysis or review
We are unaware of any such meta-analysis or review which compare different dose of mannitol for brain tumor surgery.
AUTHORS’ CONCLUSIONS
Implications for practice
TXA is beneficial in reducing the volume of blood loss. However, this does not always translate to less blood transfusion.
Implications for research
Further research to assess (1) the most effective dose and (2) the timing of TXA administration is needed, as they are among the points not covered by this meta-analysis.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Bagwe S, Chung LK, Lagman C, Voth BL, Barnette NE, Elhajjmoussa L. Blood transfusion indications in neurosurgical patients: A systematic review. Clin Neurol Neurosurg. 2017. 155: 83-9
2. Bharath K, Bhagat H, Mohindra S. Use of tranexamic acid as a rescue measure to achieve hemostasis after massive blood loss in a pediatric neurosurgical patient. J Neurosurg Anesthesiol. 2011. 23: 376-7
3. Calapai G. Systematic review of tranexamic acid adverse reactions. J Pharmacovigi. 2015. 3: 4
4. Carabini LM, Moreland NC, Vealey RJ, Bebawy JF, Koski TR, Koht A. A randomized controlled trial of low-dose tranexamic acid versus placebo to reduce red blood cell transfusion during complex multilevel spine fusion surgery. World Neurosurg. 2018. 110: e572-9
5. Cheriyan T, Maier SP, Bianco K, Slobodyanyuk K, Rattenni RN, Lafage V. Efficacy of tranexamic acid on surgical bleeding in spine surgery: A meta-analysis. Spine J. 2015. 15: 752-61
6. Choi HY, Hyun SJ, Kim KJ, Jahng TA, Kim HJ. Effectiveness and safety of tranexamic acid in spinal deformity surgery. J Korean Neurosurg Soc. 2017. 60: 75-81
7. D’Errico CC, Munro HM, Buchman SR, Wagner D, Muraszko KM. Efficacy of aprotinin in children undergoing craniofacial surgery. J Neurosurg. 2003. 99: 287-90
8. Dadure C, Sauter M, Bringuier S, Bigorre M, Raux O, Rochette A. Intraoperative tranexamic acid reduces blood transfusion in children undergoing craniosynostosis surgery. Anesthesiology. 2011. 114: 856-61
9. Dunn CJ, Goa KL. Tranexamic acid: A review of its use in surgery and other indications. Drugs. 1999. 57: 1005-32
10. 11. Efficacy of Tranexamic Acid in Brain Tumor Resections Full Text View. Available from: https://www.clinicaltrials.gov/ct2/show/NCT01655927 [Last accessed on 2020 Aug 28]. 12. Fodstad H, Forssell A, Liliequist B, Schannong M. Antifibrinolysis with tranexamic acid in aneurysmal subarachnoid hemorrhage: A consecutive controlled clinical trial. Neurosurgery. 1981. 8: 158-65 13. Furtmüller R, Schlag MG, Berger M, Hopf R, Huck S, Sieghart W. Tranexamic acid, a widely used antifibrinolytic agent, causes convulsions by a γ-aminobutyric acidA receptor antagonistic effect. J Pharmacol Exp Ther. 2002. 301: 168-73 14. Grant JA, Howard J, Luntley J, Harder J, Aleissa S, Parsons D. Perioperative blood transfusion requirements in pediatric scoliosis surgery. J Pediatr Orthop. 2009. 29: 300-4 15. Gruenbaum SE, Ruskin KJ. Red blood cell transfusion in neurosurgical patients. Curr Opin Anaesthesiol. 2014. 27: 470-3 16. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011. 343: d5928 17. Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M.editors. Cochrane Handbook for Systematic Reviews of Interventions. United States: John Wiley and Sons; 2019. p. 18. Hillman J, Fridriksson S, Nilsson O, Yu Z, Säveland H, Jakobsson KE. Immediate administration of tranexamic acid and reduced incidence of early rebleeding after aneurysmal subarachnoid hemorrhage: A prospective randomized study. J Neurosurg. 2002. 97: 771-8 19. Hooda B, Chouhan RS, Rath GP, Bithal PK, Suri A, Lamsal R. Effect of tranexamic acid on intraoperative blood loss and transfusion requirements in patients undergoing excision of intracranial meningioma. J Clin Neurosci. 2017. 41: 132-8 20. Hooda B, Muthuchellappan R. Tranexamic acid in neuroanesthesia and neurocritical care: Time for its critical appraisal. J Neuroanaesth Crit Care. 2019. 6: 257-66 21. Jellish WS, Murdoch J, Leonetti JP. Perioperative management of complex skull base surgery: The anesthesiologist’s point of view. Neurosurg Focus. 2002. 12: e5 22. Karkouti K, Wijeysundera DN, Yau TM, Beattie WS, Abdelnaem E, McCluskey SA. The independent association of massive blood loss with mortality in cardiac surgery. Transfusion. 2004. 44: 1453-62 23. Krishnan G, Panda N, Wig J, Singla N, Mukherjee K, Aluhwalia J. Effect of tranexamic acid on blood loss and the quality of surgical field in meningioma resection surgery. J Neuroanesthesiol Crit Care. 2015. 2: 157-8 24. Lelubre C, Bouzat P, Crippa IA, Taccone FS. Anemia management after acute brain injury. Crit Care. 2016. 20: 152 25. Lonjaret L, Guyonnet M, Berard E, Vironneau M, Peres F, Sacrista S. Postoperative complications after craniotomy for brain tumor surgery. Anaesth Crit Care Pain Med. 2017. 36: 213-8 26. Meneghini L, Zadra N, Aneloni V, Metrangolo S, Faggin R, Giusti F. Erythropoietin therapy and acute preoperative normovolaemic haemodilution in infants undergoing craniosynostosis surgery. Pediatr Anesth. 2003. 13: 392-6 27. Novikov V, Kondrat’ev A, Driagina N, Nazarov R. Using of tranexamic acid (Tranexam) for prevention and correction of coagulopathy during brain tumors removal. Anesteziol Reanimatol. 2011. 4: 61-6 28. Perel P, Salman RA, Kawahara T, Morris Z, Prieto-Merino D, Roberts I. CRASH-2 (clinical randomisation of an antifibrinolytic in significant haemorrhage) intracranial bleeding study: The effect of tranexamic acid in traumatic brain injury a nested, randomised, placebo-controlled trial. Health Technol Assess. 2012. 16: 3-12 29. Rajagopalan V, Chouhan RS, Pandia MP, Lamsal R, Rath GP. Effect of intraoperative blood loss on perioperative complications and neurological outcome in adult patients undergoing elective brain tumor surgery. J Neurosci Rural Pract. 2019. 10: 631-40 30. Rolston JD, Han SJ, Lau CY, Berger MS, Parsa AT. Frequency and predictors of complications in neurological surgery: National trends from 2006 to 2011: Clinical article. J Neurosurg. 2014. 120: 736-45 31. Seddighi AS, Motiei-Langroudi R, Sadeghian H, Moudi M, Zali A, Asheghi E. Factors predicting early deterioration in mild brain trauma: A prospective study. Brain Inj. 2013. 27: 1666-70 32. Shakeri M, Salehpour F, Shokouhi G, Aeinfar K, Aghazadeh J, Mirzaei F. Minimal dose of tranexamic acid is effective in reducing blood loss in complex spine surgeries: A randomized double-blind placebo controlled study. Asian Spine J. 2018. 12: 484-9 33. Shi J, Luo D, Weng H, Zeng X, Lin L, Chu H. Optimally estimating the sample standard deviation from the five-number summary. Res Synth Methods. 2020. 11: 641-54 34. Siddiqui A, Abbas H. Use of tranexamic acid to reduce intraoperative bleeding in craniotomy for meningioma patients. Anesth Analg. 2018. 126: 384-6 35. Soltani F, Nozar N, Mehdi K, Amir S, Fatemeh JF, Reza BI. Assessment of the effect of tranexamic acid on intraoperative blood loss in patients undergoing craniotomy for tumor excision. JAP. 2017. 8: 61-70 36. Sutanto S, Bisri DY, Bisri T. Effects of intravenous tranexamic acid on blood loss and transfusion requirements in tumor removal surgery of suspected meningioma. J Neuroanestesi Indones. 2019. 8: 8-16 37. Udupi B, Prakash MS, Adinarayanan S, Mishra S, Babu L, Vel R. Effect of low dose tranexamic acid on intra-operative blood loss in neurosurgical patients. Saudi J Anaesth. 2015. 9: 42 38. Vel R, Udupi BP, Prakash MV, Adinarayanan S, Mishra S, Babu L. Effect of low dose tranexamic acid on intra-operative blood loss in neurosurgical patients. Saudi J Anaesth. 2015. 9: 42-8 39. Wang Y, Liu S, He L. Prophylactic use of tranexamic acid reduces blood loss and transfusion requirements in patients undergoing cesarean section: A meta-analysis. J Obstet Gynaeco Res. 2019. 45: 1562-75 40. Yutthakasemsunt S, Kittiwatanagul W, Piyavechvirat P, Thinkamrop B, Phuenpathom N, Lumbiganon P. Tranexamic acid for patients with traumatic brain injury: A randomized, double-blinded, placebo-controlled trial. BMC Emerg Med. 2013. 2013: 13-20 41. Zehtabchi S, Abdel Baki SG, Falzon L, Nishijima DK. Tranexamic acid for traumatic brain injury: A systematic review and meta-analysis. Am J Emerg Med. 2014. 32: 1503-9 42. Zhang F, Wang K, Li FN, Huang X, Li Q, Chen Z. Effectiveness of tranexamic acid in reducing blood loss in spinal surgery: A meta-analysis. BMC Musculoskelet Disord. 2014. 15: 448