- Department of Anesthesiology, Advocate Illinois Masonic Medical Center, Chicago, Illinois, USA
- Ghaly Neurosurgical Associates, Aurora, Illinois, USA
- Department of Anesthesiology, University of Illinois, Chicago, Illinois, USA
Correspondence Address:
Ramsis F. Ghaly
Department of Anesthesiology, Advocate Illinois Masonic Medical Center, Chicago, Illinois, USA
Department of Anesthesiology, University of Illinois, Chicago, Illinois, USA
DOI:10.4103/sni.sni_132_18
Copyright: © 2018 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Ramsis F. Ghaly, Thomas Zouki, Aby Pynadath, Kenneth D. Candido, Nebojsa Nick Knezevic. Transforaminal epidural steroid injection can result in further neurological injury in a patient with severe foraminal stenosis and nerve impingement. 10-Aug-2018;9:159
How to cite this URL: Ramsis F. Ghaly, Thomas Zouki, Aby Pynadath, Kenneth D. Candido, Nebojsa Nick Knezevic. Transforaminal epidural steroid injection can result in further neurological injury in a patient with severe foraminal stenosis and nerve impingement. 10-Aug-2018;9:159. Available from: http://surgicalneurologyint.com/surgicalint-articles/transforaminal-epidural-steroid-injection-can-result-in-further-neurological-injury-in-a-patient-with-severe-foraminal-stenosis-and-nerve-impingement/
Abstract
Background:Chronic low back pain (LBP) is highly prevalent and costly in our society. The use of epidural steroid injections (ESIs) for the treatment of radicular LBP is very widespread and continues to rise. The most popular injection is the lumbar/sacral transforaminal epidural steroid injection (TFESI). Here, we present a serious neurological complication resulting from such a TFESI that was only reversed by timely neurosurgical intervention.
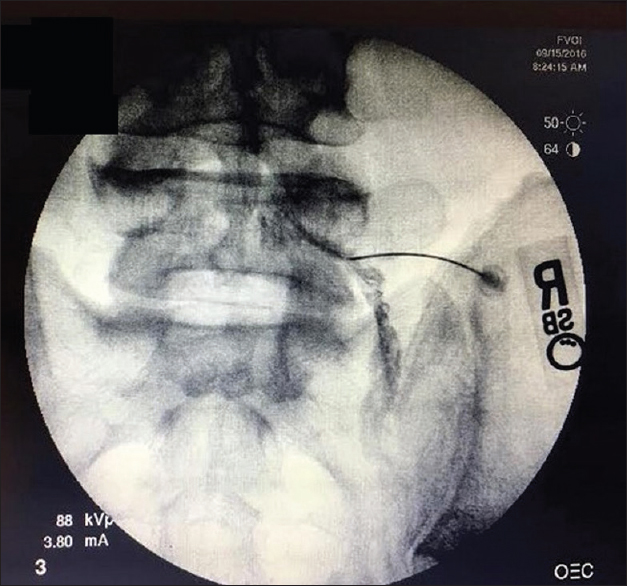
Case Description:A 49-year-old male presented with a 5-year history of progressive neurogenic claudication and right lower extremity pain/radiculopathy. He had previously received multiple lumbar ESIs and other conservative therapy. Due to a recent exacerbation of his radiculopathy associated with MRI-documented lumbosacral spondylosis, he underwent a right L5/S1 TFESI under fluoroscopic guidance. This resulted in acute right lower extremity weakness accompanied by a right-sided foot drop and sphincter dysfunction. Although the follow-up MRI was noncontributory, the EMG showed L5/S1 denervation, and the patient underwent an L4–5, L5–S1 laminectomy with discectomies at the L4–5 and L5–S1 levels. Immediately after the surgery, the patient's weakness and sensory deficits improved. Two years later, the patient continued to do well without evidence of recurrence of signs or symptoms of lumbosacral radiculopathy.
Conclusion:Patients should be counseled about the risk and benefits of TFESI. Surgical treatment may be warranted in patients who develop acutely progressive worsening following these non-FDA (Food/Drug Administration) approved injections.
Keywords: Chronic, complications, discectomy, foraminotomy, laminectomy, low back pain, non-FDA (Food/Drug Administration) approved, transforaminal epidural steroid injections
INTRODUCTION
Many patients with degenerative lumbar disc disease (DDD) are treated with non-FDA (Food/Drug Administration) approved transforaminal epidural steroid injection (TFESI). In theory, TFESIs have the advantage of resulting in greater flow into the anterior epidural space versus midline ILESI approach that predominantly results in posterior flow. However, TFESIs are correlated with various major adverse events that are typically unreported or underreported, and include: spinal cord infarction, paralysis, weakening of discs, and discitis.[
CASE REPORT
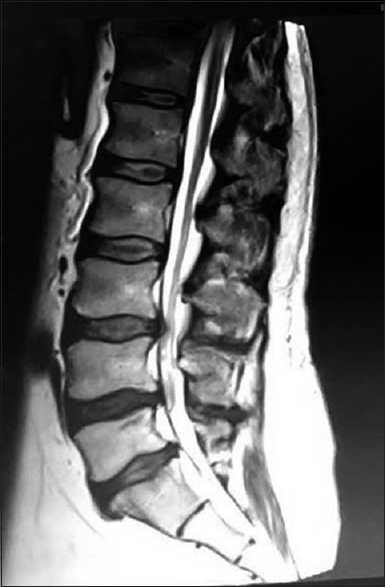
A 49-year-old male presented with a 5-year history of progressive neurogenic claudication and right more than left lower extremity L4–S1 radiculopathy. The lumbar MRI showed significant disc herniations at the L4–5 and L5–S1 levels contributing to moderate central/foraminal stenosis [
DISCUSSION
Despite the recent increase in the number of TFESI being performed, the true incidence of complications is unknown as these are largely unreported or underreported. Here we present a major neurologic deficit resulting from an L5–S1 TFESI as consequence of direct nerve root/spinal cord injury, and/or vascular injury.
Vascular insult
Intra-arterial injection of particulate steroids (insoluble steroid) or direct arterial injury has been described as potential causes of devastating neurological injuries resulting from TFESI. Kennedy et al. reported two cases of bilateral lower extremity paralysis with neurogenic bowel/bladder dysfunction following lumbar TFESI.[
Direct nerve injury and spinal cord injury
In the current case, dexamethasone, a nonparticulate steroid was used and resulted in nerve root rather than a spinal cord injury. The authors attributed this patient's neurological deficit to an acute increase in mass effect attributed to the volume of injectate resulting in ischemia. In the lumbar region, acute forceful injection of a solution into a neural foramen can lead to further entrapment of a compromised nerve root. Furthermore, an inadvertent intraneural injection cannot be ruled out. Other pathology, such an acute epidural abscess would take a longer period to become symptomatic.[
Lessons learned and avoidance of complications
The available literature shows conflicting results regarding the superior efficacy of TFESI versus ILESI for back pain of any cause, and further note their lack of FDA approval for safety/efficacy in the spine at any level.[
Notably, we would recommend TFESI be avoided when there is evidence of acute/subacute worsening of neurologic symptoms/signs. Furthermore, patients undergoing TFESI should be told about its potential risks and benefits, along with the lack of FDA approval for insufficient documentation of safety/efficacy. In all cases, one should employ the smallest dose possible, and avoid an intra-arterial injection; of interest, a negative aspiration does not guarantee that the needle is not intravascular.[
CONCLUSIONS
Lumbar TFESI had no documented long-term safety/efficacy and are not FDA approved for use in the spine at any level. Furthermore, the risks/complications are typically unreported or underreported. Here we present a patient who following an L5–S1 TFESI developed acute right-lower extremity numbness/weakness/foot drop, and benefited from emergent laminectomy/surgical intervention, recovering full preoperative function.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Candido KD, Raghavendra MS, Chinthagada M, Badiee S, Trepashko DW. A prospective evaluation of iodinated contrast flow patterns with fluoroscopically guided lumbar epidural steroid injections: The lateral parasagittal interlaminar epidural approach versus the transforaminal epidural approach. AnesthAnalg. 2008. 106: 638-44
2. Chang Chien GC, Candido KD, Knezevic NN. Digital subtraction angiography does not reliably prevent paraplegia associated with lumbar transforaminal epidural steroid injection. Pain Physician. 2012. 15: 515-23
3. Furman MB, O’Brien EM, Zgleszewski TM. Incidence of intravascular penetration in transforaminal lumbosacral epidural steroid injections. Spine. 2000. 25: 2628-32
4. Gharibo CG, Fakhry M, Diwan S, Kaye AD. Conus medullaris infarction after a right L4 transforaminal epidural steroid injection using dexamethasone. Pain Physician. 2016. 19: E1211-4
5. Houten , JK , Errico TJ. Paraplegia after lumbosacral nerve root block: Report of three cases. Spine J. 2002. 2: 70-5
6. Huntoon MA, Martin DP. Paralysis after transforaminal epidural injection and previous spinal surgery. RegAnesth Pain Med. 2004. 29: 494-5
7. Kennedy DJ, Dreyfuss P, Aprill CN, Bogduk N. Paraplegia Following Image-Guided Transforaminal Lumbar Spine Epidural Steroid Injection: Two Case Reports. Pain Med. 2009. 10: 1389-94
8. Knezevic NN, Lissounov A, Candido KD. Transforaminal vs interlaminar epidural steroid injections: Differences in the surgical rates and safety concerns. Pain Med. 2014. 15: 1975-6








Sharon Craver
Posted April 8, 2019, 11:38 am
I have had several Lumbar steroid injections and was never told they were “non-FDA approved”. After having more problems following the procedures, I started looking things up. Particularly, I had one in June, 2018. One week later, I was diagnosed with meningitis (viral) which upon studying this that if it’s viral, it could be that it was just lying in wait in the nerve endings waiting to attack. That’s what I believe happened to me. I called the doctor who supposedly did the injection who said there was no relation in the incidences. I was also upset because this was a “teaching hospital” and one I was prepped and lying still on the table, I heard some people outside in a hallway laughing and cutting up. My doctor told me that he was having his “associate” actually perform the procedure. I could not tell who was doing it but it was the most painful procedure that I had ever had. Of course the week later when I had meningitis, the students and doctors had to lumbar puncture me 4-times to get spinal fluid and it was EXTREMELY painful. So with that said, I will never have another one even though I am worse off than ever.
Now I am having numbness in my left leg all the way down to my heel. I was just going to tough it out but was worried about permanent damage. I have another MRI done and it showed that due to further stenosis, the sciatic nerve is definitely pinched. The doctor who told me this said my back was in bad shape due to stenosis, spondylosis, scoliosis and disk problems it would make any type of surgery extremely difficult and it was not recommended.
So now I am worried about my future as I am not that old and cannot just sit down now. I have too much to do.
Also, I would like to mention that your report states that problems are unreported or under-reported. That may be because the doctors think they would be admitting that they are the cause of the problem because of it being a “non-FDA approved” procedure.
What are your thoughts. My doctor just blew me off like I should not be complaining. …and I am not a complainer.
Brad
Posted November 13, 2019, 8:26 am
I would call a lawyer
I am kindoff experiencing the same thing
I’m worried about arachnoiditis from these damn shots
I’ve had five