- Department of Neurosurgery, University of Colorado School of Medicine, Aurora, United States,
- Department of Anesthesiology, University of Colorado School of Medicine, Aurora, United States.
Correspondence Address:
Keanu Chee, Department of Neurosurgery, University of Colorado School of Medicine, Aurora, Colorado, United States.
DOI:10.25259/SNI_21_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ashkaun Razmara1, Shaquia Idlett-Ali1, Keanu Chee1, Keshari Shrestha1, Eric Bayman1, John Thompson1, Leslie Jameson2, Steven Ojemann1, Daniel Kramer1. Transient cardiac asystole during vagus nerve stimulator implantation: A case report. 08-Apr-2022;13:131
How to cite this URL: Ashkaun Razmara1, Shaquia Idlett-Ali1, Keanu Chee1, Keshari Shrestha1, Eric Bayman1, John Thompson1, Leslie Jameson2, Steven Ojemann1, Daniel Kramer1. Transient cardiac asystole during vagus nerve stimulator implantation: A case report. 08-Apr-2022;13:131. Available from: https://surgicalneurologyint.com/surgicalint-articles/11519/
Abstract
Background: Vagal nerve stimulation (VNS) is a Food and Drug Administration approved therapy for seizures with a suggested mechanism of action consisting of cortical desynchronization, facilitated through broad release of inhibitory neurotransmitters in the cortex and brainstem. The vagus nerve contains visceral afferents that transmit sensory signals centrally, from locations that include the heart and the aorta. Although the vagus nerve serves a role in cardiac function, electrical stimulation with VNS has rarely resulted in adverse cardiac events. Here, we report a case of a cardiac event during left-sided VNS implantation.
Case Description: A 22-year-old male with an 8-year history of absence seizures and a 3-year history of medically refractory generalized tonic-clonic seizure was planned for surgical implantation of a VNS device. In the operating room, the patient underwent left-sided VNS implantation. An initial impedance check was performed with subsequent wound irrigation; following a few seconds of irrigation, a 5 s complete cardiac pause was noted. A repeated impedance check, which included turning on the stimulation, did not replicate the cardiac pause. No further pauses or cardiac events were noted and the case continued to completion without issue. The patient was later activated without any further complications.
Conclusion: This report describes the initiation of a cardiac event, unlikely resulting from VNS, but instead time linked to intraoperative irrigation directly on the vagus nerve.
Keywords: Cardiac event, Electrical stimulation, Epilepsy, Vagus nerve, Vagus nerve stimulator
INTRODUCTION
Vagal nerve stimulation (VNS) was approved by the Food and Drug Administration (FDA) in 1997, for the treatment of partial seizures in patients aged 12 years and older. The device consists of coiled stimulation leads placed around the vagus nerve. VNS has demonstrated an average reduction in seizure frequency of at least 50% in over half of patients[
The vagus nerve, or tenth cranial nerve, is composed primarily of afferent visceral fibers transmitting visceral sensory information from receptors in the heart, aorta, lungs, and gastrointestinal system to the central nervous system (CNS). These fibers project to the nucleus solitarius located in the medulla,[
The commonly reported side effects of VNS include voice alteration, hoarseness, throat or neck pain, headache, cough, and dyspnea.[
CASE PRESENTATION
The patient is a 22-year-old right-handed male who presented to clinic for the evaluation of adjunctive therapy for medically refractory epilepsy. His seizures began at the age of 14 and were described as blank staring spells. One year later, he was diagnosed with absence (generalized nonmotor) seizures and started on pharmacologic therapy. After exploring different medications, a therapeutic regimen consisting of lamotrigine (Lamictal) 300 mg twice daily and lacosamide (Vimpat) 200 mg twice daily achieved seizure freedom for the following 4 years. However, at the age of 19, he experienced his first generalized tonic-clonic seizure (GTCS). Following the onset of GTCS at 19 years old, the patient now has two-to-three GTCS annually and furthermore has not had concomitant absence seizures.
The patient described seizure onset as feeling lightheaded, shaky, and having the need to sit down. In most cases, he is typically unable to reach a sitting position before losing balance from the onset of convulsion. The seizure lasts approximately 2 min, and it takes him up to 6 min to regain awareness. Witnesses report the occurrence of speech vocalizations during these events.
Despite compliance with his medication regimen, breakthrough unprovoked seizures continued, including once while driving. The patient has no other significant or relevant medical history. Epilepsy conference review determined that this patient was a good candidate for VNS and was subsequently scheduled to undergo left-sided VNS implantation with SenTiva battery placement.
In the operating room, dissection of the neck proceeded in the usual fashion. Briefly, the carotid sheath was opened over the carotid artery, vagus nerve, and internal jugular vein. The vagus nerve was identified and isolated for approximately 2–2.5 cm of nerve. A blue background was placed underneath the nerve. The wires were then wrapped around the vagus nerve. Once the leads were secured, the battery was placed inside the chest wall. Per standard protocol, a small volume of irrigation was then applied over the nerve and wires, and the system was appropriately interrogated with electrical stimulation. From this assessment, the system was noted to have good impedance (1820 Ohms) without any change in heart rate. The internal heart rate sensor was then utilized, at which point no further stimulation (i.e., impedance checking) occurred. This took approximately 2 min, and the heart rate was noted to be stable during this time.
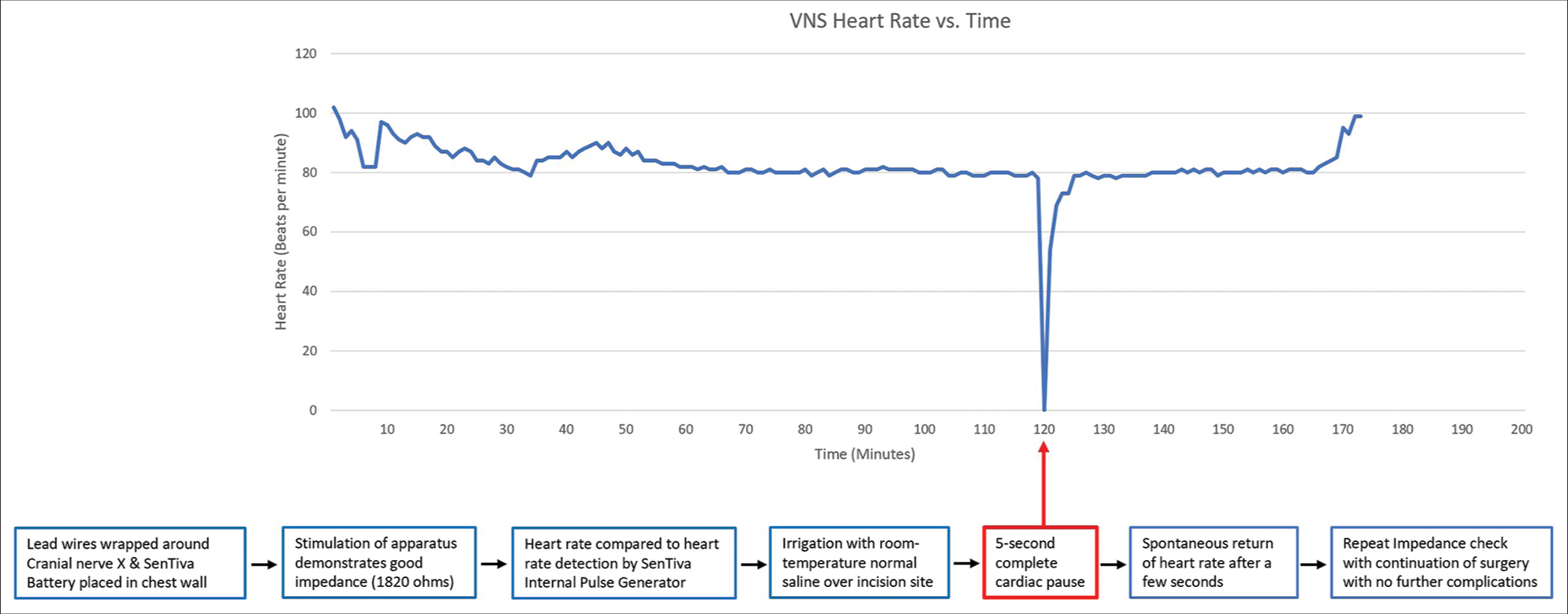
The wound was further irrigated with room temperature sterile normal saline with retractors in place and the nerve exposed. A few seconds into irrigation, the anesthesiologist promptly reported a cessation of the heart rate and informed the surgical team who subsequently stopped surgery [
Figure 1:
Initial impedance check of vagus nerve stimulation device demonstrated proper function and the internal heart rate sensor showed a normal heart rate. Following routine irrigation of the wound with normal saline, there was a 5 s complete cardiac pause noted on heart rate monitoring. After this brief period of asystole, there was spontaneous recovery in heart rate with no further cardiac events through completion of the operation.
DISCUSSION
Here, we report the case of a cessation of heart rate following irrigation directly on the vagus nerve. Although the nerve had been stimulated with an impedance check, the stimulation was off for a minimum of 2 full min before the event, and during the stimulation, no immediate changes were noted. The timing, however, was aligned to bolus irrigation directly striking the vagus nerve, which was exposed due to the positioning of the retractors and thus appears to be an event due to mechanical stimulation.
The intricate relationship of the vagus nerve to cardiac function originally raised concern that VNS may affect cardiac rhythm and function. The effects of electrical stimulation of the cervical vagus nerve in animal models have been shown to have a modest effect on heart rate, blood pressure, and the gastrointestinal system.[
The autonomic effects previously described in animal models are rarely seen in clinical human application. The previous studies report the incidence of bradyarrhythmias with VNS to be about 0.1%.[
Prior studies have suggested potential reasons for asystole during intraoperative lead testing including abnormal electrode placement, indirect stimulation of the cervical cardiac nerves, technical malfunction of the device, and polarity reversal of the leads.[
Simple irrigation with room temperature sterile saline directly over the vagus nerve was sufficient to stimulate vagal visceral efferent fibers and produces a brief cardiac pause in this patient. To the authors’ knowledge, this is the first case of this nature to be reported in the literature. Several other explanations were considered such as irrigation hitting the carotid bifurcation, stimulation of baroreceptors located in the carotid sinus, which could have caused the hemodynamic changes seen. However, it was much more likely due to irrigating directly over the vagus nerve as the carotid artery was not exposed or visible in the surgical field.
On review of the intraoperative anesthesia record, the cardiac pause was seen a few seconds after the initiation of irrigation. Although the saline had been kept at room temperature, it felt slightly cold to touch, which may have contributed to the reaction seen. In addition, the nerve was manipulated regularly in the placement of the electrodes without any notable reactions or events, as is usually the case in this procedure. Thus, the mechanical stimulation was more likely related to repeated pulsation of irrigation on the nerve. Of note, the patient and his mother did report that he has a strong vagal response and gets lightheaded at the sight of blood; whether this is significant to the case is unclear. Ultimately, the case presented demonstrates a rarely seen phenomenon. Although recommendations for such a rare event would be difficult to make, it is also a simple matter to not allow the irrigation to strike the nerve directly during VNS placement.
CONCLUSION
VNS represents an important adjunctive therapy in the treatment of medically refractory epilepsy. The intricate relationship of the vagus nerve to cardiac function raises concern that VNS may affect cardiac rhythm and function. We present the rare event of a cardiac pause after intraoperative irrigation directly on the vagus nerve during VNS implantation.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Agostoni E, Chinnock JE, De Daly MB, Murray JG. Functional and histological studies of the vagus nerve and its branches to the heart, lungs and abdominal viscera in the cat. J Physiol. 1957. 135: 182-205
2. Ali II, Pirzada NA, Kanjwal Y, Wannamaker B, Medhkour A, Koltz MT. Complete heart block with ventricular asystole during left vagus nerve stimulation for epilepsy. Epilepsy Behav. 2004. 5: 768-71
3. Ardesch JJ, Buschman HP, van der Burgh PH, Wagener-Schimmel LJ, van der Aa HE, Hageman G. Cardiac responses of vagus nerve stimulation: Intraoperative bradycardia and subsequent chronic stimulation. Clin Neurol Neurosurg. 2007. 109: 849-52
4. Asconapé JJ, Moore DD, Zipes DP, Hartman LM, Duffell WH. Bradycardia and asystole with the use of vagus nerve stimulation for the treatment of epilepsy: A rare complication of intraoperative device testing. Epilepsia. 1999. 40: 1452-4
5. Barnes A, Duncan R, Chisholm JA, Lindsay K, Patterson J, Wyper D. Investigation into the mechanisms of vagus nerve stimulation for the treatment of intractable epilepsy, using 99mTc-HMPAO SPET brain images. Eur J Nucl Med Mol Imaging. 2003. 30: 301-5
6. Ben-Menachem E. Vagus nerve stimulation, side effects, and long-term safety. J Clin Neurophysiol. 2001. 18: 415-8
7. Cechetto DF. Central representation of visceral function. Fed Proc. 1987. 46: 17-23
8. Galli R, Limbruno U, Pizzanelli C, Giorgi FS, Lutzemberger L, Strata G. Analysis of RR variability in drug-resistant epilepsy patients chronically treated with vagus nerve stimulation. Auton Neurosci Basic Clin. 2003. 107: 52-9
9. Hamlin RL, Smith CR. Effects of vagal stimulation on S-A and A-V nodes. Am J Physiol. 1968. 215: 560-8
10. Liu C, Jiang H, Yu L, Po S. Vagal stimulation and arrhythmias. J Atr Fibrillation. 2020. 13: 2398
11. Maier SF, Goehler LE, Fleshner M, Watkins LR.editors. The role of the vagus nerve in cytokine-to-brain communication. Annals of the New York Academy of Sciences. Hoboken, New Jersey: Blackwell Publishing Inc; 1998. 840: 289-300
12. Morris GL, Gloss D, Buchhalter J, Mack KJ, Nickels K, Harden C. Evidence-based guideline update: Vagus nerve stimulation for the treatment of epilepsy: Report of the guideline development subcommittee of the american academy of neurology. Neurology. 2013. 81: 1453-9
13. Murphy JV. Left vagal nerve stimulation in children with medically refractory epilepsy. J Pediatr. 1999. 134: 563-6
14. Rutecki P. Anatomical, physiological, and theoretical basis for the antiepileptic effect of vagus nerve stimulation. Epilepsia. 1990. 31: S1-6
15. Tatum WO, Moore DB, Stecker MM, Baltuch GH, French JA, Ferreira JA. Ventricular asystole during vagus nerve stimulation for epilepsy in humans. Neurology. 1999. 52: 1267-7
16. Uthman BM, Reichl AM, Dean JC, Eisenschenk S, Gilmore R, Reid S. Effectiveness of vagus nerve stimulation in epilepsy patients: A 12-year observation. Neurology. 2004. 63: 1124-6
17. Woodbury DM, Woodbury JW. Effects of vagal stimulation on experimentally induced seizures in rats. Epilepsia. 1990. 31: S7-19