- Department of Neurosurgery, Kanazawa Medical Center, Kanazawa, Japan.
DOI:10.25259/SNI_187_2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Yu Shimizu, Katsuhiro Tsuchiya, Hironori Fujisawa. Transient ischemic attack in elderly patient with PHACE syndrome. 27-Sep-2019;10:188
How to cite this URL: Yu Shimizu, Katsuhiro Tsuchiya, Hironori Fujisawa. Transient ischemic attack in elderly patient with PHACE syndrome. 27-Sep-2019;10:188. Available from: http://surgicalneurologyint.com/surgicalint-articles/9675/
Abstract
Background: Posterior fossa brain malformations, hemangiomas, arterial anomalies, coarctation of the aorta and cardiac defects, and eye abnormalities (PHACE) is a rare congenital anomaly with a broad spectrum of clinical manifestations.
Case Description: We describe a 75-year-old male with PHACE anomaly, aortic anomaly, malformation of brain, aplastic right carotid artery, and cervical vasculopathy. He presented with a transient ischemic attack with the left hemiparesis, a rare clinical presentation of the PHACE syndrome. He had an uneventful recovery and recently completed a 2-year follow-up after the superficial temporal artery to middle cerebral artery anastomosis.
Conclusion: PHACE syndrome should be kept in mind, even in individuals of advanced age, in the instance of a TIA, especially in situations which may involve induced hypoperfusion.
Keywords: Aplasia of carotid artery, Dandy–Walker syndrome, PHACE syndrome, Transient ischemic attack
INTRODUCTION
PHACE is a neurocutaneous syndrome including malformations of the posterior fossa, facial hemangiomas, arterial anomalies, cardiac anomalies, aortic coarctation, and abnormalities of the eye.[
CASE REPORT
A 75-year-old male presented with sudden onset of weakness in his left upper and lower extremities. Medical history confirmed that the patient was a full-term birth without trauma or history of hypoxia. Hemangioma on the right side of the neck was noted after birth. Routine laboratory examinations were unremarkable. Neuropsychological evaluation was normal.
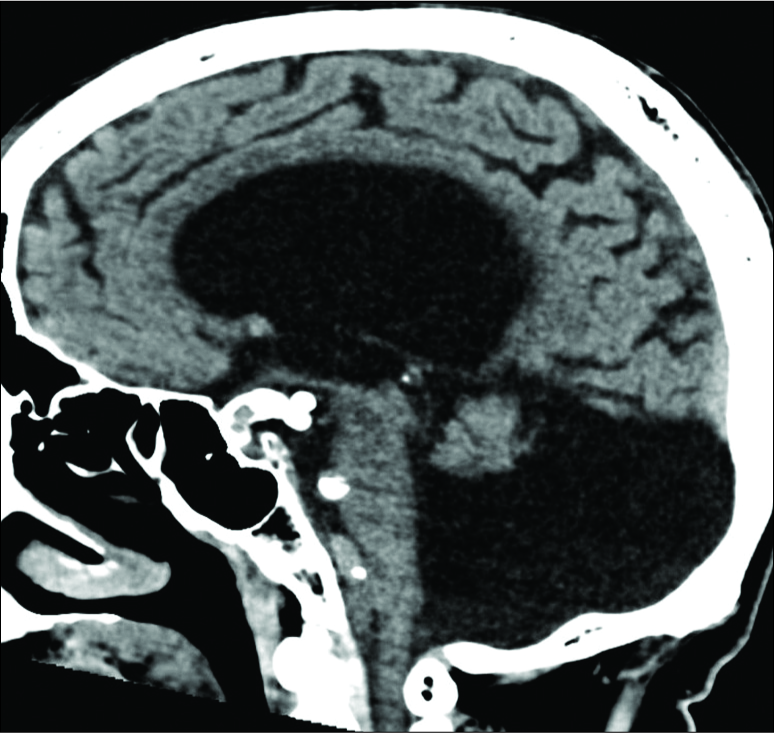
Magnetic resonance imaging of the brain revealed multiple posterior fossa anomalies including agenesis of the cerebellar vermis with hydrocephalus and hypoplasia of the right cerebellar vermis. Accordingly, the patient was diagnosed with Dandy–Walker malformation [
Figure 2:
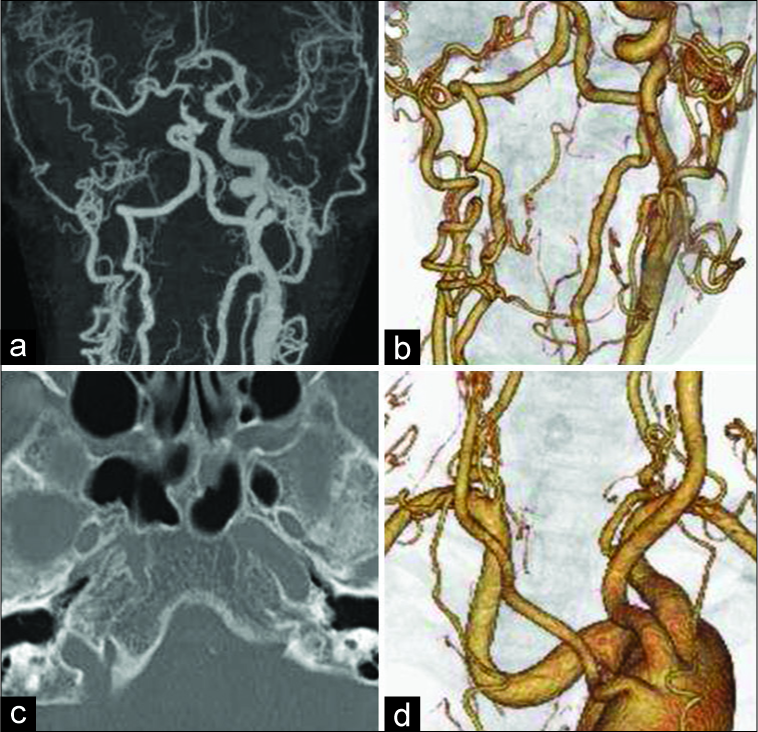
(a) Maximum intensity projections of computed tomography (CT) angiography demonstrate stenosis of the basilar artery and aplasia of the right common carotid artery. (b) Multiplanar reconstruction of CT angiography of the neck demonstrates aplasia of the right internal carotid artery. (c) Skull base CT scanning demonstrates an absence of the right carotid canal. (d) Abnormal origin and course of the left subclavian vessels are demonstrated. Bilateral common carotid arteries have arisen from the distal side of the left subclavian artery.
Anomalous aortic arch anatomy was also seen. The left subclavian artery arose from the medial wall of the aortic arch, more proximal than expected. Bilateral common carotid arteries arose from the distal side of the left subclavian artery. The right subclavian artery had an aberrant course, with its proximal portion making a U-shaped bend [
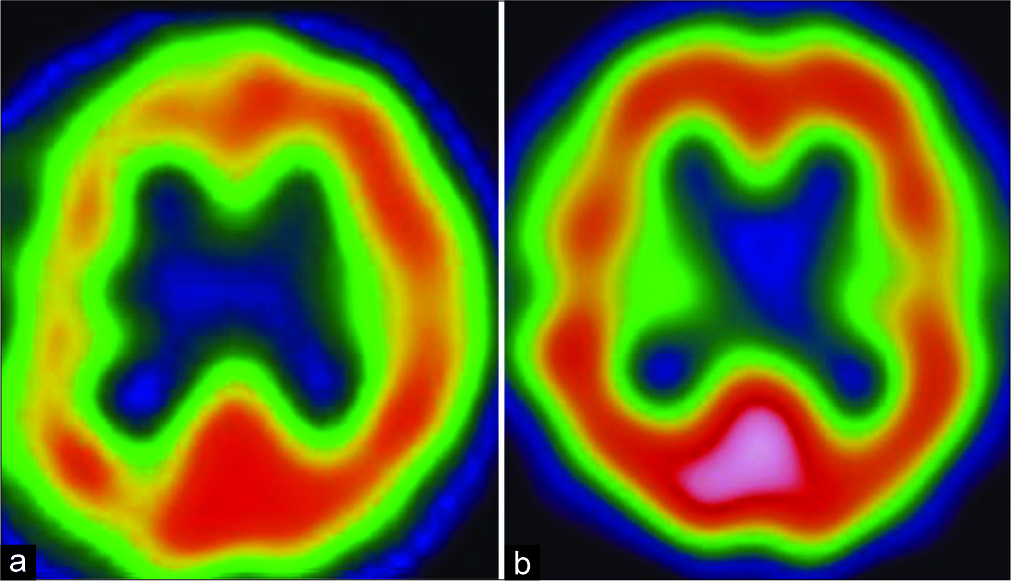
Single-photon emission computed tomography (SPECT) demonstrated reduced cerebral blood flow (CBF) in the border zones between the middle and posterior cerebral artery territories of the right hemisphere [
Figure 3:
(a) Single-photon emission computed tomography demonstrates reduced cerebral blood flow in the border zones between the middle and posterior cerebral artery territories in the right hemisphere. (b) After extracranial–intracranial bypass, cerebral blood flow of the right hemisphere improves to the normal level.
DISCUSSION
PHACE syndrome was first reported in 1996. The acronym stands for a set of characteristic disorders that include posterior fossa malformations, hemangioma, arterial anomalies, coarctation of the aorta or cardiac defects, and eye abnormalities.[
Our patient presented at 75 years of age, and PHACE was not considered earlier when he was found to have a Dandy–Walker malformation. It is important to emphasize that a complete imaging evaluation be conducted in patients, even if they are of advanced age. In this patient, a new diagnosis of PHACE syndrome was given, according to the diagnostic criteria presented by Sullivan et al.[
CONCLUSION
PHACE syndrome is a rare congenital neurocutaneous syndrome. It usually presents during infancy and childhood. Here we present a rare case of adult PHACE syndrome with aplasia of the ICA. The patient experienced TIA which induced ipsilateral hypoperfusion. PHACE syndrome should be kept in mind, even in individuals of advanced age, in the instance of a TIA, especially in situations which may involve induced hypoperfusion. Details of birth history, complete surveys of the brain, and intracranial and extracranial vasculatures are strongly advised because these patients are at increased risk of TIA.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Alberti A, Paciaroni M, Caso V, Venti M, Palmerini F, Agnelli G. Early seizures in patients with acute stroke: Frequency, predictive factors, and effect on clinical outcome. Vasc Health Risk Manag. 2008. 4: 715-20
2. Arora SS, Plato BM, Sattenberg RJ, Downs RK, Remmel KS, Heidenreich JO. Adult presentation of PHACES syndrome. Interv Neuroradiol. 2011. 17: 137-46
3. Burrows PE, Robertson RL, Mulliken JB, Beardsley DS, Chaloupka JC, Ezekowitz RA. Cerebral vasculopathy and neurologic sequelae in infants with cervicofacial hemangioma: Report of eight patients. Radiology. 1998. 207: 601-7
4. Frieden IJ, Reese V, Cohen D. PHACE syndrome. The association of posterior fossa brain malformations, hemangiomas, arterial anomalies, coarctation of the aorta and cardiac defects, and eye abnormalities. Arch Dermatol. 1996. 132: 307-11
5. Garzon MC, Epstein LG, Heyer GL, Frommelt PC, Orbach DB, Baylis AL. PHACE syndrome: Consensus-derived diagnosis and care recommendations. J Pediatr. 2016. 178: 24-3300
6. Haggstrom AN, Garzon MC, Baselga E, Chamlin SL, Frieden IJ, Holland K. Risk for PHACE syndrome in infants with large facial hemangiomas. Pediatrics. 2010. 126: e418-26
7. Heyer GL, Dowling MM, Licht DJ, Tay SK, Morel K, Garzon MC. The cerebral vasculopathy of PHACES syndrome. Stroke. 2008. 39: 308-16
8. Metry D, Heyer G, Hess C, Garzon M, Haggstrom A, Frommelt P. Consensus statement on diagnostic criteria for PHACE syndrome. Pediatrics. 2009. 124: 1447-56
9. Metry DW, Dowd CF, Barkovich AJ, Frieden IJ. The many faces of PHACE syndrome. J Pediatr. 2001. 139: 117-23
10. Metry DW, Haggstrom AN, Drolet BA, Baselga E, Chamlin S, Garzon M. A prospective study of PHACE syndrome in infantile hemangiomas: Demographic features, clinical findings, and complications. Am J Med Genet A. 2006. 140: 975-86
11. Siegel DH, Shieh JTC, Kwon EK, Baselga E, Blei F, Cordisco M. Copy number variation analysis in 98 individuals with PHACE syndrome. J Invest Dermatol. 2013. 133: 677-84
12. Sullivan CT, Christian SL, Shieh JT, Metry D, Blei F, Krol A. X chromosome-inactivation patterns in 31 individuals with PHACE syndrome. Mol Syndromol. 2013. 4: 114-8