- Department of Neurosurgery, National Hospital Organization Beppu Medical Center, Beppu, Japan.
- Department of Neurosurgery, Oita Prefectural Hospital, Oita, Japan.
- Department of Neurosurgery, Faculty of Medicine, Oita University, Yufu, Oita, Japan.
Correspondence Address:
Mitsuhiro Anan
Department of Neurosurgery, Faculty of Medicine, Oita University, Yufu, Oita, Japan.
DOI:10.25259/SNI_750_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Mitsuhiro Anan1, Yasuyuki Nagai2, Takeshi Matsuda1, Minoru Fujiki3. Trauma may affect vasa vasorum to promote thrombosis and enlargement of intracranial aneurysms: A case report. 13-Jan-2021;12:16
How to cite this URL: Mitsuhiro Anan1, Yasuyuki Nagai2, Takeshi Matsuda1, Minoru Fujiki3. Trauma may affect vasa vasorum to promote thrombosis and enlargement of intracranial aneurysms: A case report. 13-Jan-2021;12:16. Available from: https://surgicalneurologyint.com/surgicalint-articles/10523/
Abstract
Background: Thrombosed intracranial aneurysm (IA) is likely to occur in large or giant IAs. Almost all thrombosed IAs are found already in a thrombosed state, and few reports have depicted the process of thrombosis in unthrombosed aneurysm. Moreover, no reports appear to have described IA in which thrombosis accelerated after trauma.
Case Description: We report herein a case in which an unthrombosed large cerebral aneurysm rapidly thrombosed and grew within 3 months after trauma. The highlight in this unusual case was that during surgery, the aneurysm and anterior skull base were adherent and some blood vessels bridged between the aneurysm and dura mater. Histologically, intramural hemorrhage was seen in the tunica media of the aneurysm.
Conclusion: Trauma may act as a “second hit” causing adhesion between IAs and surrounding tissues, accelerating inflammation of the vasa vasorum and aneurysmal walls, and thrombosis in IAs.
Keywords: Intracranial aneurysm, Large aneurysm, Thrombosed aneurysm, Trauma, Vasa vasorum
INTRODUCTION
Thrombosed intracranial aneurysms (IAs) are often found only once they have already become relatively large.[
CASE REPORT
History and examination
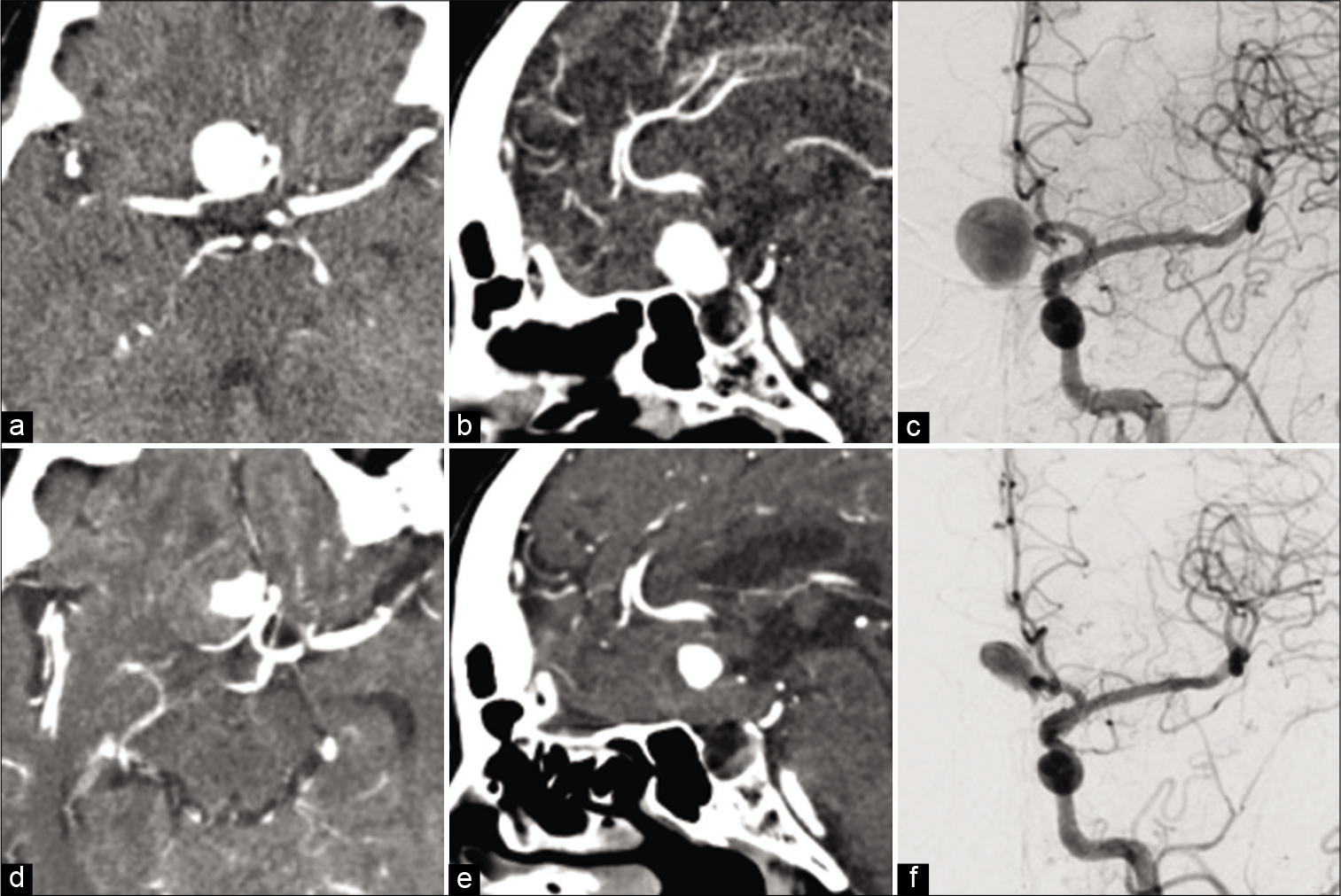
A 78-year-old woman was admitted to our hospital due to a traffic accident, presenting with mild traumatic brain injury, neck pain, rib fracture, and lumbar fracture. Computed tomography (CT) of the head revealed no traumatic lesions such as skull fracture, subarachnoid hemorrhage, or brain contusion, but a 19 mm mass lesion was identified at the base of the right frontal lobe. CT angiography and digital subtraction angiography (DSA) revealed a right anterior cerebral artery aneurysm with a dome measuring 19.0 × 14.7 mm and a neck 4.7 mm in diameter [
Figure 1:
Radiological examinations on presentation and at the 3-month follow-up. The initial computed tomography (CT) shows aneurysm at the base of the anterior fossa with homogeneous enhancement on axial view (a) and sagittal view (b). Digital subtraction angiography (DSA) shows cerebral aneurysm of the right anterior cerebral artery (proximal A2 portion) on the left angiography (c). CT at the 3-month follow-up shows that the aneurysm has grown and is partially thrombosed on contrast-enhanced CT (d and e) and DSA (f).
Operation
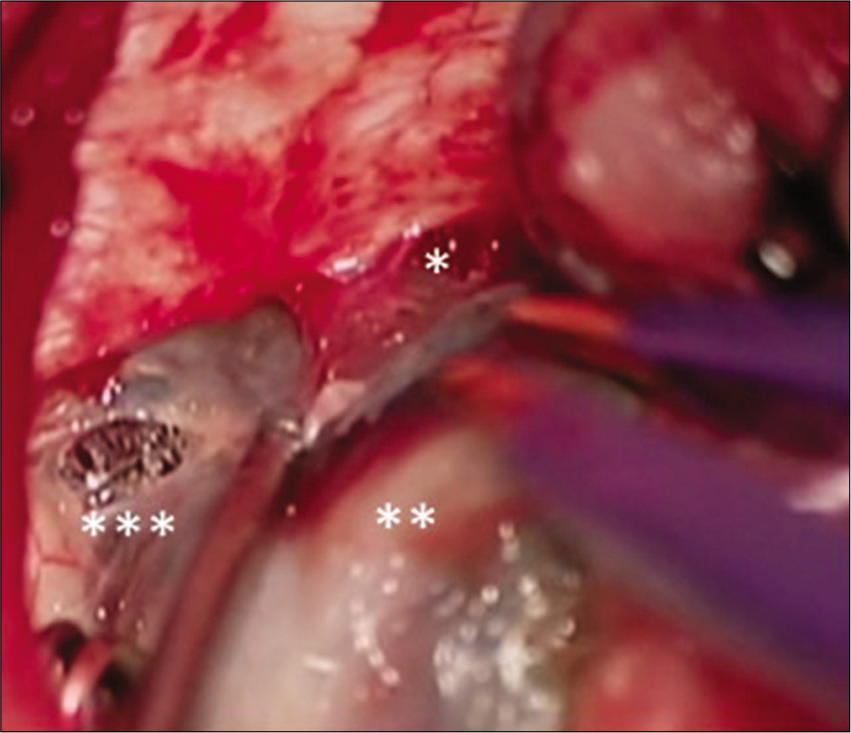
The aneurysm was approached under an interhemispheric approach. Since the aneurysm was hard and could not be directly clipped due to thrombosis, A3-A3 in situ bypass and trapping were performed. The anteroinferior part of the aneurysm was adherent to the anterior skull base, and multiple microvessels were seen bridging between the aneurysm wall and dura mater [
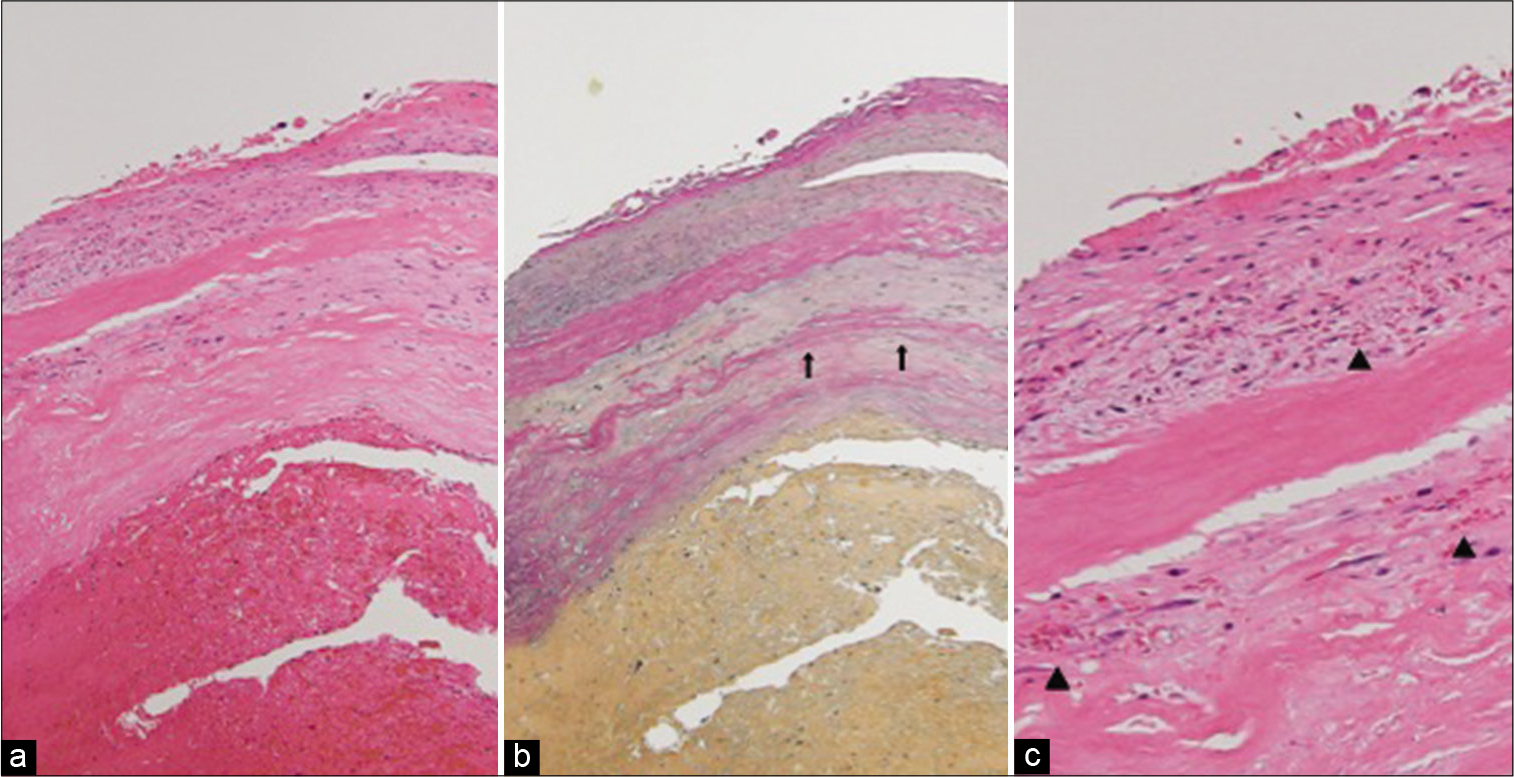
Histology of the aneurysm
Endothelial cells and the internal elastic lamina of the aneurysm had partially disappeared, the media were thick and the smooth muscle layer had degenerated and disappeared in parts. Intramural hemorrhage was identified on the outside of the tunica media [
Postoperative course
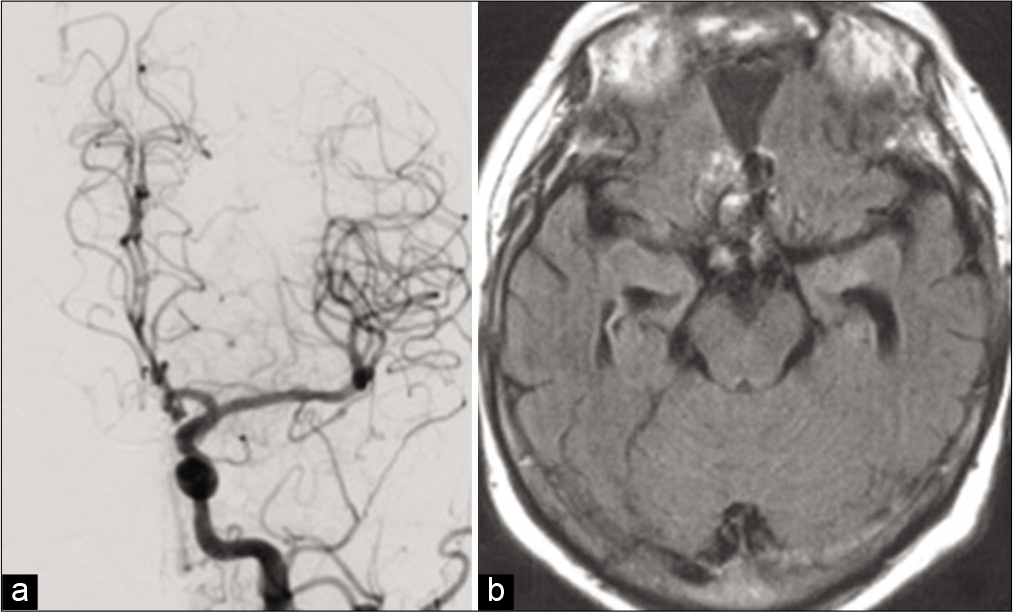
The aneurysm was not apparent on DSA and showed reduced volume on MR imaging [
DISCUSSION
One of the factors influencing thrombosis in unthrombosed IAs is the form of the aneurysm. As the aspect ratio (depth/ neck width) increases, the internal blood flow inside is slowed, and thrombus is thus more likely to form in the IA.[
The highlight in this unusual case was that during surgery, the aneurysm and dura mater were adherent and some bridging blood vessels were identified between the two. Regarding the relationship between thrombosed cerebral aneurysms and the vasa vasorum, few reports have examined the mechanisms of aneurysm growth with interrupted blood circulation.[
A key limitation in this study was the lack of ways to determine whether neovessels had been present between the aneurysm and dura of the skull base before trauma. As this report only examined a single case, further studies of larger cohorts are still needed.
CONCLUSION
Trauma may promote thrombosis and growth of IAs by accelerating inflammatory conditions of the aneurysmal walls.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors wish to thank Dr. Koji Yoshikawa for his advice on pathology.
References
1. Aoki T, Frösen J, Fukuda M, Bando K, Shioi G, Tsuji K. Prostaglandin E2-EP2-NF-κB signaling in macrophages as a potential therapeutic target for intracranial aneurysms. Sci Signal. 2017. 10: eaah6037
2. Atlas SW, Grossman RI, Goldberg HI, Hackney DB, Bilaniuk LT, Zimmerman RA. Partially thrombosed giant intracranial aneurysms: Correlation of MR and pathologic findings. Radiology. 1987. 162: 111-4
3. Crawford T. Some observations on the pathogenesis and natural history of intracranial aneurysms. J Neurol Neurosurg Psychiatry. 1959. 22: 259-66
4. Frösen J, Tulamo R, Paetau A, Laaksamo E, Korja M, Laakso A. Saccular intracranial aneurysm: Pathology and mechanisms. Acta Neuropathol. 2012. 123: 773-86
5. Iihara K, Murao K, Sakai N, Soeda A, Ishibashi-Ueda H, Yutani C. Continued growth of and increased symptoms from a thrombosed giant aneurysm of the vertebral artery after complete endovascular occlusion and trapping: The role of vasa vasorum. Case report. J Neurosurg. 2003. 98: 407-13
6. Kataoka H, Yagi T, Ikedo T, Imai H, Kawamura K, Yoshida K. Hemodynamic and histopathological changes in the early phase of the development of an intracranial aneurysm. Neurol Med Chir (Tokyo). 2020. 60: 319-28
7. Koyama S, Kotani A, Sasaki J. Giant basilar artery aneurysm with intramural hemorrhage and then disastrous hemorrhage: Case report. Neurosurgery. 1996. 39: 174-7
8. Krings T, Alvarez H, Reinacher P, Ozanne A, Baccin CE, Gandolfo C. Growth and rupture mechanism of partially thrombosed aneurysms. Interv Neuroradiol. 2007. 13: 117-26
9. Lawton MT, Quiñones-Hinojosa A, Chang EF, Yu T. Thrombotic intracranial aneurysms: Classification scheme and management strategies in 68 patients. Neurosurgery. 2005. 56: 441-54
10. Nagahiro S, Takada A, Goto S, Kai Y, Ushio Y. Thrombosed growing giant aneurysms of the vertebral artery: Growth mechanism and management. J Neurosurg. 1995. 82: 796-801
11. Portanova A, Hakakian N, Mikulis DJ, Virmani R, Abdalla WM, Wasserman BA. Intracranial vasa vasorum: Insights and implications for imaging. Radiology. 2013. 267: 667-79
12. Schunk H. Spontaneous thrombosis of intracranial aneurysms. Am J Roentgenol Radium Ther Nucl Med. 1964. 91: 1327-38
13. Stehbens WE. Histopathology of cerebral aneurysms. Arch Neurol. 1963. 8: 272-85
14. Takaba M, Endo S, Kurimoto M, Kuwayama N, Nishijima M, Takaku A. Vasa vasorum of the intracranial arteries. Acta Neurochir (Wien). 1998. 140: 411-6
15. Ujiie H, Tachibana H, Hiramatsu O, Hazel AL, Matsumoto T, Ogasawara Y. Effects of size and shape (aspect ratio) on the hemodynamics of saccular aneurysms: A possible index for surgical treatment of intracranial aneurysms. Neurosurgery. 1999. 45: 119-29
16. Whittle IR, Dorsch NW, Besser M. Spontaneous thrombosis in giant intracranial aneurysms. J Neurol Neurosurg Psychiatry. 1982. 45: 1040-7
17. Yasui T, Sakamoto H, Kishi H, Komiyama M, Iwai Y, Yamanaka K. Rupture mechanism of a thrombosed slow-growing giant aneurysm of the vertebral artery-case report. Neurol Med Chir (Tokyo). 1998. 38: 860-4