- Department of Neurology and Neurosurgery, Universidade Federal de São Paulo, Sao Paulo, Brazil.
Correspondence Address:
Leonardo Favi Bocca, Department of Neurology and Neurosurgery, Universidade Federal de São Paulo, Sao Paulo, Brazil.
DOI:10.25259/SNI_490_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Leonardo Favi Bocca, João Vitor Fernandes Lima, Italo Capraro Suriano, Sergio Cavalheiro, Thiago Pereira Rodrigues. Traumatic acute subdural hematoma and coma: retrospective cohort of surgically treated patients. 24-Aug-2021;12:424
How to cite this URL: Leonardo Favi Bocca, João Vitor Fernandes Lima, Italo Capraro Suriano, Sergio Cavalheiro, Thiago Pereira Rodrigues. Traumatic acute subdural hematoma and coma: retrospective cohort of surgically treated patients. 24-Aug-2021;12:424. Available from: https://surgicalneurologyint.com/surgicalint-articles/11059/
Abstract
Background: A subdural hematoma is defined as clot formation in the subdural space after vessel rupture or brain parenchyma damage. Several demographic and tomographic factors were associated to poor prognosis, although some debate according to their specific roles still remains.
Methods: Retrospective cohort study of comatose patients admitted to a single-institution, tertiary hospital center, between the years 2013 and 2019 with traumatic acute subdural hematoma requiring surgical evacuation were studied. Demographic and tomographic data were obtained from medical records. Univariate and multivariate statistical analysis were performed, using a value of P
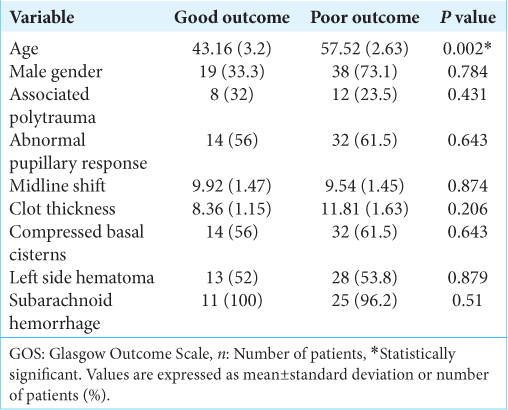
Results: Seventy-seven patients were selected using the criteria and a total of 37 (48%) head CT exams were evaluated. The overall mortality was 57.1% and achieved 100% at ≥75-years-old subgroup. Univariate analysis only found young age as a good prognosis factor (P = 0.002). Gender (P = 0.784), abnormal pupillary response (P = 0.643), midline shift (P = 0.874), clot thickness (P = 0.206), compressed basal cisterns (P = 0.643), hematoma side (P = 0.879), and subarachnoid hemorrhage (P = 0.510) showed no association. Multivariate analysis showed no statistically significant association between covariates.
Conclusion: Traumatic acute subdural hematoma is a life-threatening condition. Younger age was the only positive prognostic factor identified. More research is necessary to establish age as a rule-out criterion to surgical indication.
Keywords: Acute subdural hematoma, Coma, Prognosis, Trauma
INTRODUCTION
Traumatic brain injury (TBI) is the major contributor to death and disability among all traumatic injuries[
The TBI is classically divided into “closed” and “open,” the latter resulting from the loss of integrity of the dura mater.[
A multitude of scores and scales can be used to classify the TBI severity, ranging from neuropathological classifications, clinical scales, and neuropsychological tests.[
The exact incidence of acute subdural hematoma is unknown; however, 12–29% of patients with severe TBI have acute subdural hematoma in the head computed tomography (CT) exams, with an incidence of 11% when pooling all admissions for TBI (mild, moderate, and severe).[
The clinical manifestations of acute subdural hematoma may vary, ranging from an initial GCS of 8 or less, that is, coma, from 37% to 80% of patients admitted to the hospital, to cases with a “lucid interval,” when after a transient loss of consciousness, a period of neurological normality is established, followed by progressive deterioration and coma, from 12% to 38% of patients.[
Head CT is the diagnostic study of choice for acute traumatic injuries as it allows an adequate diagnosis of associated brain and skull injuries and outlines the treatment of the patient.[
Even when present as isolated lesions, acute subdural hematomas have a poor prognosis, with mortality rates varying in the literature from 27% to up to 60%.[
The surgical treatment for patients with acute subdural hematoma is based on initial GCS score, pupillary examination, comorbidities, tomographic findings, age, and elevated intracranial pressure in the monitored patients.[
Several clinical and demographic risk factors for progression to a poor outcome, such as death or severe disability at hospital discharge, have already been reported in the literature and some independent makers on head CT exam led to a worse prognosis, including hematoma thickness, midline deviation, basal cisterns compression and the presence of associated subarachnoid hemorrhage.[
Despite being relatively frequent in the scenario of severe TBI, the prognostic factors related to the demographic, clinical and radiological data of patients are still not fully understood in some subgroups of patients such as the elderly.[
MATERIALS AND METHODS
Study design
We performed a retrospective cohort study of patients admitted to the Emergency Department a single-institution, tertiary hospital center, between the years 2013 and 2019 who received traumatic acute subdural hematoma (International Statistical Classification of Diseases and Related Health Problems, ICD-10 S06.5) as the main diagnosis, being clinically in a coma (GCS less than or equal to 8 points) and who underwent surgical treatment of the hematoma by craniotomy or craniectomy. Indication of surgery and clot evacuation with or without decompressive craniectomy was made by an assistant neurosurgeon at hospital admission. There is not a standardized institutional protocol guiding surgical technique indication, but midline shift higher than hematoma thickness, brain bulging out skull after dural opening and bilateral intraparenchymal hematomas were indications to decompressive craniectomy. However, when decompressive craniectomy is performed, the institutional protocol requires large skull flap (at least 14 × 12 cm), dural opening with pericranium duroplasty and temporal bone squamous part removal in all patients.
Inclusion and exclusion criteria
All patients older than 18 years, with a primary diagnosis of traumatic acute subdural hematoma (ICD-10 S06.5) and GCS less than or equal to 8 points at admission (prehospital care or previous hospital care referred), with at least one head CT exam before surgery were enrolled in the study. Patients without surgical treatment or <1-year of follow-up were excluded from this study.
Ethical considerations
Ethical approval was obtained from the Institutional Ethical Committee (registered under CAAE 40234920.4.0000.5505, March 01, 2021). As a retrospective cohort study with results showed as pooled data, patient’s consent was waived by the ethical committee.
Data collection and measure procedure
Glasgow Outcome Scale (GOS) score at 1-year follow-up was the primary outcome of this study. Patients with a GOS score of 4 or 5 were classified as a good functional outcome and those with a score of 1–3 were grouped as poor functional outcome. Data was obtained retrospectively from the patient’s medical record and the head CT performed preoperatively. Demographic data (age and gender), medical history, other traumas (polytrauma), and pupillary light response were obtained from the patient’s medical record. Tomographic data from the initial image (hematoma side, compressed basal cisterns, midline shift at the septum pellucidum in millimeters, thickness of the hematoma in millimeters, presence of subarachnoid hemorrhage, and other associated bleeding injuries) were obtained from and the measure done using the RADIANT software version 2020.2 64-bit by one senior author. The outpatient functional outcome data were obtained from the patient’s medical record up to 12 months after discharge and classified according to the GOS scale. Before the statistical analysis, each patient received a random identification number anonymizing the sample data.
Data analysis
Descriptive statistics (means with standard deviation for continuous data and frequencies with proportions for categorical data) were presented for the recovered data. For univariate analysis, continuous variables were compared with Student’s t test for data with normal distribution, while categorical variables were analyzed by Pearson’s Chi-square test or Fisher’s exact test when comparing groups (good and bad prognosis). We removed from statistical analysis patients without head CT measures when performing univariate analysis.
A multivariate logistic regression model was performed to identify the variables that were independently associated with the worst outcome, with adjustment for age, gender, mortality, functional status at discharge, and tomographic variables.
For the statistically significant association, a value of P < 0.05 was used. All analyses were performed using the statistical program IBM Statistical Package for the Social Sciences version 26.
RESULTS
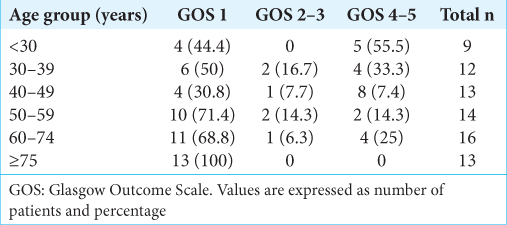
A total of 121 patients were admitted to our emergency department with a main diagnosis of traumatic acute subdural hematoma between 2013 and 2019 and underwent clot evacuation surgery, with or without craniectomy. From this group, 77 patients presented comatose to our first evaluation (GCS 8 or less) and they were included in this study. Twenty-seven (35%) patients had GCS 3 at first care. Because our hospital receives referred patients from all metropolitan area and there is no digital integration among all hospitals, many patients underwent surgery without repeating head CT at our hospital, limiting the number of head CT exams able to be evaluated. A total of 37 (48%) head CT exams were evaluated and underwent statistical analysis.
During hospital care, 44 (57.1%) patients evolved to death and only 19 (24.7%) patients were classified as good prognosis, or independent, at 1 year follow-up [
The association between younger age and good prognosis was statistically significant (P = 0.002). All other variables studied, gender (P = 0.784), abnormal pupillary response to light (P = 0.643), midline shift (P = 0.874), clot thickness (P = 0.206), compressed basal cisterns (P = 0.643), hematoma side (P = 0.879), and associated subarachnoid hemorrhage (P = 0.510) showed no statistically significant association to prognosis.
A binomial logistic regression was performed to verify the effects of age, gender, multiple system trauma, pupillary response, midline shift, clot thickness, compressed basal cisterns, subarachnoid hemorrhage, and hematoma side on the likelihood that participants have a bad prognosis. For this study, only individuals with head CT measured variables were selected. The logistic regression model was not statistically significant, χ2(9) = 15,994, P = 0.067 [
DISCUSSION
Traumatic acute subdural hematoma is associated with severe TBI in 12–30% of patients[
Similar to overall literature, we found a mortality rate of 57.1% and only 24.7% of patients were independent at hospital discharge. Our lower good functional outcome compared to literature can be explained by our worse initial GCS score (only severe TBI patients included).
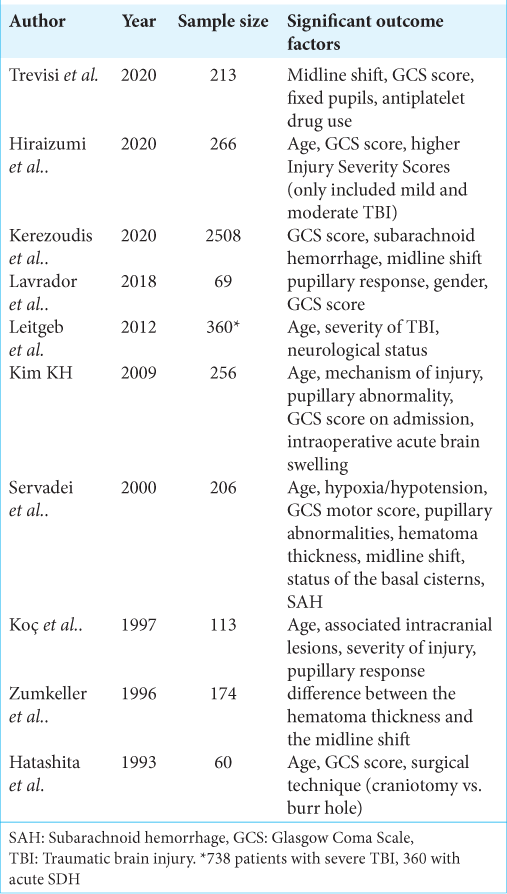
Demographic factors that negatively affect prognosis reported include advanced age,[
Some factors are still under discussion, as the worse prognosis by male patients showed by Lavrador et al.[
The most recent consensus[
Identification of prognostic factors helps clinical decision-making, especially when this decision needs to be made in minutes, and based on data obtained in an emergency care setting and with the patient himself being unable to express his opinion. Thus, the existence of representative outcome data can assist in the decision process made between the assistant team and the patient’s family. Supporting the high likelihood of acceptance to decompressive surgery when indicated by an assistant physician, Clark et al. asked acceptability to be enrolled into RESCUE-ASDH trial. Results found 96% and 91% acceptance even when surrogate consent by their next of kin and an independent doctor, respectively, were the person doing the decision.[
The retrospective nature of our study was an important limitation. Other data regarding CT parameters and more detailed record’s information could improve establishment of certainty of results. Despite indication to surgery was done using current protocols, the surgical technique (clot evacuation with or without craniectomy) used varied between neurosurgeons and may contribute to prognosis. Regarding prognosis, most patients were referred to our center from other secondary hospitals and their prognosis may have been affected by the time between trauma and treatment at our institution. Finally, as our center limitation, about 52% of head CT exams were unavailable to be evaluated, limiting the statistical power of our results.
CONCLUSION
Traumatic acute subdural hematoma is a life-threatening condition. Despite adequate surgical treatment, its mortality and morbidity rates are significant. The most reliable attitude to decrease its consequences remains on prophylactic measures, as to all other TBI complications.
Even among severe TBI cases on this study, younger age was the only independent prognostic factor contributing to good prognosis. More research is needed to establish imaging and demographic markers as definitive prognostic factors and possibly supporting a decision-making guideline.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Allen DN, Thaler NS, Cross CL, Mayfield J.editors. Classification of traumatic brain injury severity: A neuropsychological approach. Cluster Analysis in Neuropsychological Research. New York: Springer; 2013. p. 95-123
2. Bešenski N. Traumatic injuries: Imaging of head injuries. Eur Radiol. 2002. 12: 1237-52
3. Bullock MR, Chesnut R, Ghajar J, Gordon D, Hartl R, Newell DW. Surgical management of acute subdural hematomas. Neurosurgery. 2006. 58: S16-24
4. Carroll JJ, Lavine SD, Meyers PM. Imaging of subdural hematomas. Neurosurg Clin N Am. 2017. 28: 179-203
5. Clark DJ, Kolias AG, Corteen EA, Ingham SC, Piercy J, Crick SJ. Community consultation in emergency neurotrauma research: Results from a pre-protocol survey. Acta Neurochir (Wien). 2013. 155: 1329-34
6. Dewan MC, Rattani A, Gupta S, Baticulon RE, Hung YC, Punchak M. Estimating the global incidence of traumatic brain injury. J Neurosurg. 2019. 130: 1080-97
7. Fountain DM, Kolias AG, Lecky FE, Bouamra O, Lawrence T, Adams H. Survival trends after surgery for acute subdural hematoma in adults over a 20-year period. Ann Surg. 2017. 265: 590-6
8. Gennarelli TA, Thibault LE. Biomechanics of acute subdural hematoma. J Trauma. 1982. 22: 680-6
9. Grossman RI.editors. Head trauma. Neuroradiology: The Requisites. Philadelphia, PA: Mosby; 2003. p. 243
10. Hatashita S, Koga N, Hosaka Y, Takagi S. Acute subdural hematoma: Severity of injury, surgical intervention, and mortality. Neurol Med Chir (Tokyo). 1993. 33: 13-8
11. Hiraizumi S, Shiomi N, Echigo T, Oka H, Hino A, Baba M. Factors associated with poor outcomes in patients with mild or moderate acute subdural hematomas. Neurol Med Chir (Tokyo). 2020. 60: 402-10
12. Hutchinson PJ, Kolias AG, Tajsic T, Adeleye A, Aklilu AT, Apriawan T. Consensus statement from the international consensus meeting on the role of decompressive craniectomy in the management of traumatic brain injury. Acta Neurochir (Wien). 2019. 161: 1261-74
13. Kerezoudis P, Goyal A, Puffer RC, Parney IF, Meyer FB, Bydon M. Morbidity and mortality in elderly patients undergoing evacuation of acute traumatic subdural hematoma. Neurosurg Focus. 2020. 49: E22
14. Kim KH. Predictors for functional recovery and mortality of surgically treated traumatic acute subdural hematomas in 256 patients. J Korean Neurosurg Soc. 2009. 45: 143
15. Koç RK, Akdemir H, Oktem IS, Meral M, Menkü A. Acute subdural hematoma: outcome and outcome prediction. Neurosurg Rev. 1997. 20: 239-44
16. Lavrador JP, Teixeira JC, Oliveira E, Simão D, Santos MM, Simas N. Acute subdural hematoma evacuation: predictive factors of outcome. Asian J Neurosurg. 2018. 13: 565-71
17. Leitgeb J, Mauritz W, Brazinova A, Janciak I, Majdan M, Wilbacher I. Outcome after severe brain trauma due to acute subdural hematoma. J Neurosurg. 2012. 117: 324-33
18. Maxeiner H, Wolff M. Pure subdural hematomas: A postmortem analysis of their form and bleeding points. Neurosurgery. 2002. 50: 503-9
19. Rusnak M. Giving voice to a silent epidemic. Nat Rev Neurol. 2013. 9: 186-7
20. Servadei MT, Nasi G, Giuliani F. CT prognostic factors in acute subdural haematomas: The value of the “worst” CT scan. Br J Neurosurg. 2000. 14: 110-6
21. Teasdale G, Jennett B. Assessment of coma and impaired consciousness. Lancet. 1974. 304: 81-4
22. Trevisi G, Sturiale CL, Scerrati A, Rustemi O, Ricciardi L, Raneri F. Acute subdural hematoma in the elderly: Outcome analysis in a retrospective multicentric series of 213 patients. Neurosurg Focus. 2020. 49: E21
23. Yoneoka Y, Takeda N, Inoue A, Ibuchi Y, Kumagai T, Sugai T. Human Kluver-Bucy syndrome following acute subdural haematoma. Acta Neurochir (Wien). 2004. 146: 1267-70
24. Zumkeller M, Behrmann R, Heissler HE, Dietz H. Computed tomographic criteria and survival rate for patients with acute subdural hematoma. Neurosurgery. 1996. 39: 708-12