- Department of Neurosurgery, University of California, Los Angeles,
- Department of Radiology, Harbor-UCLA Medical Center, Torrance, California, USA.
Correspondence Address:
James I. Ausman
Department of Neurosurgery, University of California, Los Angeles,
DOI:10.25259/SNI-281-2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Jason A. Chen, Matthew C. Garrett, Anton Mlikotic, James I. Ausman. Treatment of intracranial vertebral artery dissecting aneurysms involving the posterior inferior cerebellar artery origin. 25-Jun-2019;10:116
How to cite this URL: Jason A. Chen, Matthew C. Garrett, Anton Mlikotic, James I. Ausman. Treatment of intracranial vertebral artery dissecting aneurysms involving the posterior inferior cerebellar artery origin. 25-Jun-2019;10:116. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=9418
Abstract
Background: Vertebral artery dissecting aneurysm (VADA) involving the origin of the posterior inferior cerebellar artery (PICA) is a complex disease entity in which the dual goals of preventing future rebleeding and maintaining perfusion of the lateral medulla must be considered. We present an illustrative case and review the literature surrounding treatment strategies.
Case Description: We report a patient presenting with extensive subarachnoid hemorrhage due to rupture of an intracranial VADA involving the PICA origin. After consideration of the patient’s cerebral vasculature and robustness of collaterals, a flow-diverting stent was placed with angiographic resolution of the lesion and maintenance of antegrade PICA flow. Ultimately, the patient experienced a contralateral intraparenchymal hemorrhage leading to death. Review of the literature identified 124 cases of VADA involving the PICA origin described over the past decade. The methods of surgical and endovascular treatment of these cases were reviewed, with particular focus on the rationale of treatment, outcomes, and complications.
Conclusion: Numerous treatment options for VADA involving PICA have been reported with different risk and benefit profiles. Flow-diverting stents appear to offer the most favorable balance of securing the aneurysm and avoiding medullary infarction, but the risks and optimal anti-thrombotic treatment strategy are incompletely understood. In select cases, in which the surgical risk is low or in which the anatomy is favorable (e.g., nondominant parent vessel or robust collateral circulation in the involved territories), parent artery trapping with or without microsurgical revascularization can be considered.
Keywords: Flow-diverting stent, Posterior inferior cerebellar artery, Vertebral artery dissecting aneurysm
INTRODUCTION
Vertebral artery dissecting aneurysms (VADA) cause 3–5% of cases of subarachnoid hemorrhage (SAH),[
Unruptured VADA may present with headache, ischemic symptoms (e.g., lateral medullary syndrome), or mass effect, and remain stable or even improve without treatment.[
VADA is usually not amenable to treatment with standard clipping or coil embolization due to the morphology of the lesion. Involvement of branch vessels of the VA, for example, PICA, further complicates matters due to the need to preserve brainstem and cerebellar perfusion, limiting treatment options and resulting in worse outcomes.[
Here, we report an illustrative case of a patient with VADA involving the origin of PICA, with anatomically poor collateral flow to PICA demonstrated on angiography. The patient was ultimately treated with a flow-diverting stent. Treatment options and considerations for treatment of this condition were reviewed.
ILLUSTRATIVE CASE
History
A 57-year-old man with a history of hypertension and mechanical valve replacement, on warfarin, presented to Harbor-UCLA Medical Center after experiencing severe headache and decreasing level of consciousness; the precise time of onset was unknown. There was no history of preceding trauma, tobacco use, or known connective tissue disorders. By the time of initial evaluation, the patient was unresponsive and did not withdraw to noxious stimulation. The Glasgow Coma Scale score was 5 (E1V1M3), corresponding to the World Federation of Neurosurgical Societies Grade 5. His clinical condition was assessed as Hunt and Hess Grade 5. The patient’s international normalized ratio was in the therapeutic range; warfarin was held, and anticoagulation was reversed using prothrombin complex concentrate and Vitamin K.
Imaging
Noncontrast computed tomography (CT) imaging of the brain revealed diffuse hemorrhage in the subarachnoid cisterns and fourth ventricular hemorrhage (modified Fisher[
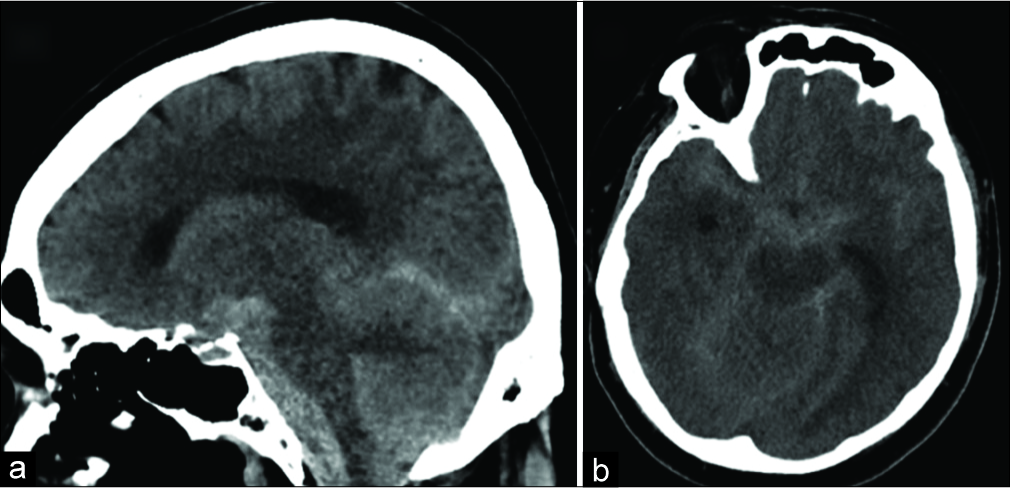
Figure 1
Noncontrast computed tomography images of the brain. (a) Axial plane, showing diffuse subarachnoid hemorrhage, especially within the prepontine cistern and foramen magnum. (b) Sagittal plane, again demonstrating subarachnoid hemorrhage. There is dilatation of the temporal horns related to secondary ventricular obstruction.
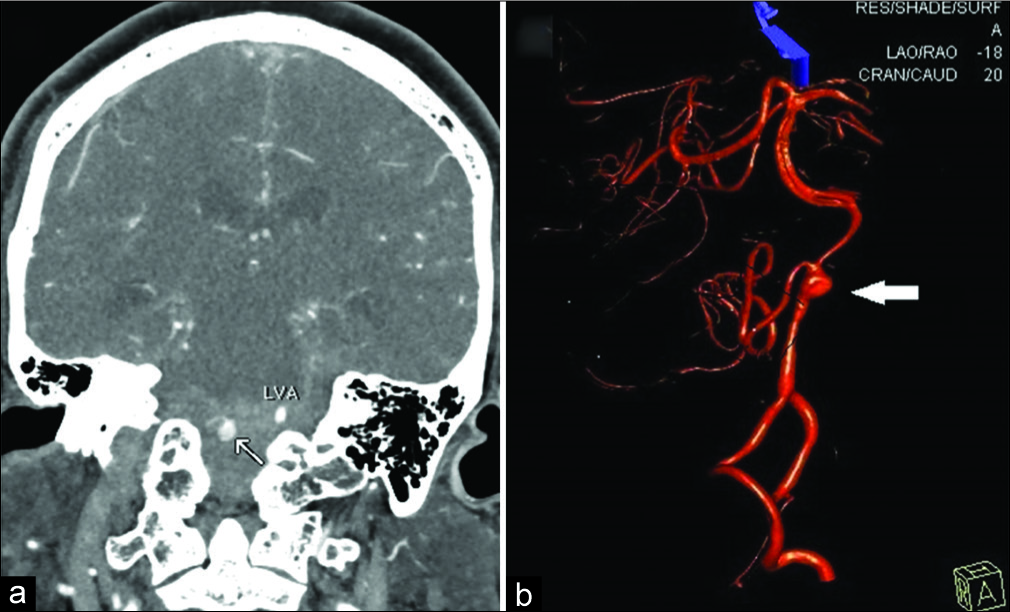
Figure 2
(a) Computed tomography angiography in the coronal plane revealed an aneurysmal lesion arising from the distal right vertebral artery (arrow) that is surrounded by high-density subarachnoid hemorrhage. The left vertebral artery (LVA) is labeled. (b) Three-dimensional volume rendered image of the distal vertebral artery during initial catheter angiography, showing vessel wall irregularity and a broad-based fusiform lesion consistent with a dissecting aneurysm (arrow) that involves a dominant posterior inferior cerebellar artery. There is also a proximal normal variant partial duplication of the vessel.
Digital subtraction angiography further revealed a dissection of the distal aspect of the vessel and a broad-based, 5 mm aneurysm that involved the origin of a large PICA [
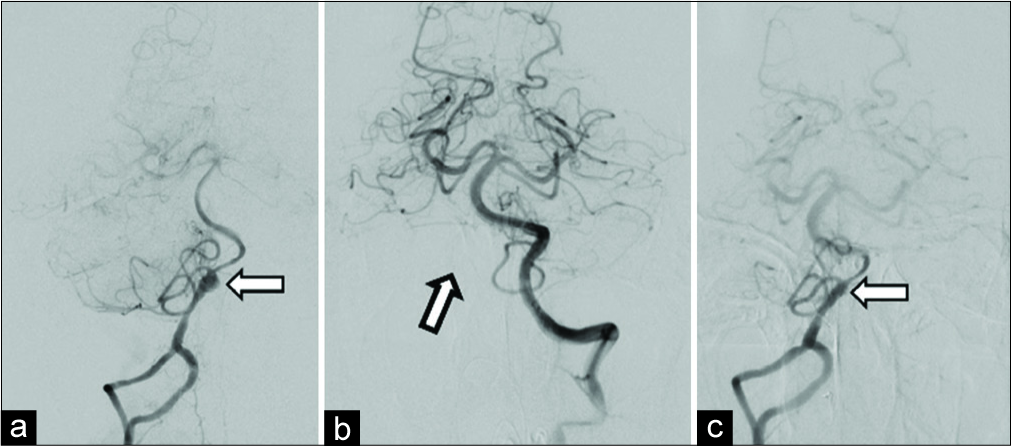
Figure 3
Digital subtraction angiography of the right vertebral artery in the anteroposterior view (a), showing a variant segmental duplication of the distal vessel, dissection, and associated aneurysm (arrow), which involves the origin of the posterior inferior cerebellar artery, and decreased flow to the distal vasculature. Left vertebral artery in the anteroposterior view (b), demonstrating a paucity of flow to the right posterior inferior cerebellar artery territory (arrow). Immediately following placement of a pipeline flow-diverting stent (c), there is an instant decrease in flow to the lesion, improved flow to the distal vasculature, and preservation of flow to the posterior inferior cerebellar artery.
Treatment
The patient was intubated and an external ventricular drain (EVD) was placed, before initiation of antiplatelet therapy. He exhibited clinical improvement with ventricular drainage to the point where he was speaking, following commands, and moving all extremities, though he was somnolent and confused. Due to the improvement in the patient’s neurological status, surgical and endovascular options were considered to (1) control the aneurysm and prevent imminent rebleeding and (2) avoid ischemia of the right PICA territory in light of lack of clear collateral perfusion. An occipital artery to PICA bypass or PICA-PICA bypass could achieve the latter objective; however, the presence of significant medical comorbidities disfavored a surgical approach. Proximal vessel sacrifice would ordinarily preserve PICA flow through retrograde flow from the left vertebral system but would perpetuate flow to the dissecting aneurysm and thus maintain the risk of rerupture. Entrapment of the lesion with proximal and distal coil embolization would protect the lesion, but antegrade flow in the PICA would be eliminated with a high risk of ischemia to the lateral medulla and cerebellum. Furthermore, it would remove a VA from the posterior circulation with unclear consequences, particularly during a period of subsequent high risk of vasospasm.
We ultimately opted to treat with the urgent placement of a flow-diverting stent (one day after initial presentation), which would with one maneuver protect the aneurysm and preserve flow through the right PICA and VA. The cell design of the stent would restore laminar flow in the native vessel, thus redirecting flow to the VA and eliminating focal turbulence that often perpetuates incompletely treated lesions. At the same time, the stent wall does not compromise flow to normal side-branching vessels (i.e., PICA). Although this is an off-label indication, we believed that it provided the best treatment solution for this patient due to the aforementioned risks of each alternative.
The patient was premedicated with a loading dose of dual antiplatelet therapy (650 mg aspirin and 600 mg clopidogrel) the day before the procedure. A 2.75 mm × 18 mm Pipeline flow-diverting stent was deployed across the dissecting aneurysm in the V4 segment of the vessel. Angiography showed slowing of flow and early partial thrombus formation in the aneurysm with preservation of flow to PICA [
Postprocedure course
The patient’s course was complicated by atrial fibrillation with rapid ventricular response and pulmonary edema on the 1st postprocedure day. His neurological status improved over subsequent days of hospitalization to the point where he was awake, responsive, following commands, and moving all extremities. He was treated for rhythm control and extubated. There was no evidence of vasospasm or rebleeding at the repaired dissecting aneurysm on repeated angiography. The patient continued on a course of 325 mg aspirin and 75 mg clopidogrel daily to prevent stent thrombosis and was observed clinically to assess platelet function. There were no signs of hyper-responsiveness to antiplatelet treatment.
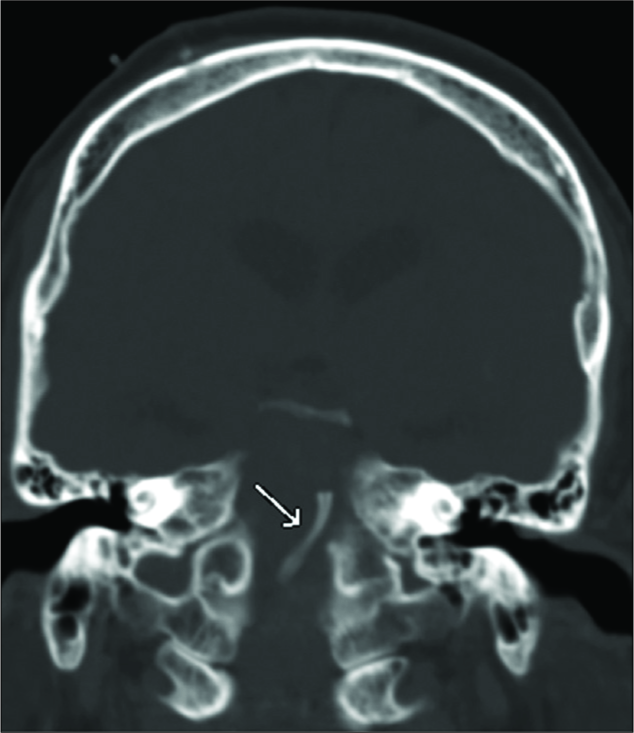
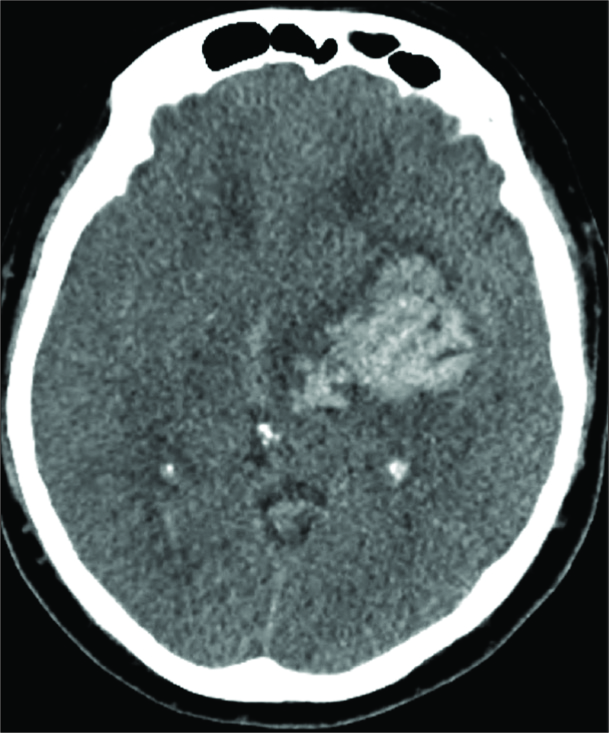
On the 10th postprocedure day, the patient developed a hemorrhage of the left thalamus and basal ganglia. An intraparenchymal hematoma measuring 6.7 cm × 5.7 cm × 5.5 cm was found on CT, with mass effect resulting in a 1 cm left-to-right midline shift and uncal herniation [
REVIEW OF LITERATURE
The treatment of VADA illustrates the importance of carefully weighing treatment risks and benefits, taking into account multiple considerations: (1) preventing further rebleeding; (2) decreasing flow/promoting thrombosis to the aneurysm; (3) maintaining perfusion of the territory of the parent vessel; (4) maintaining perfusion of the branch vessels; (5) avoiding iatrogenic complications, for example, from use of antiplatelet or anticoagulant medications. Proposed treatment options possess unique combinations of these features. Broadly, they can be classified as deconstructive, in which the segment of the parent (vertebral) artery containing the aneurysm is excluded, thereby reducing the risk of bleeding, or reconstructive, in which the dissecting aneurysm is allowed to thrombose while maintaining normal flow through the parent artery. Surgical treatments open further possibilities, in which the PICA can be revascularized, or the aneurysmal segment can be bypassed.
The database PubMed was searched using the keywords “vertebral artery dissecting aneurysm” for articles published in the past 10 years, returning 873 articles. The articles were narrowed to English-language reports of case series of VADA. Reports of single cases were excluded. Each of these articles was read to identify whether the relationship of the aneurysm to the origin of PICA was reported. In total, 160 cases from 33 articles were identified, and their histories were reviewed [
Nonoperative treatment
Experience in several cohorts indicates that VADA, when ruptured, is unstable and, if untreated, at high risk of rebleeding and subsequent mortality.[
Occasionally, nonoperative management has been attempted in patients presenting with ruptured VADA. Hashimoto et al. reported on a prospective cohort of 7 patients managed nonoperatively with sedation using fentanyl and blood pressure control using diltiazem or nicardipine, and none suffered from rebleeding in a mean 20-month follow-up time frame.[
Proximal occlusion
Endovascular and open surgical methods exist for occlusion of the VA proximal to the aneurysm. Unilateral occlusion of the VA, if it does not affect branching or perforating vessels, usually results in good neurological outcome due to the supply of the basilar artery and VA distal to the occlusion by the contralateral VA.[
In cases involving PICA, this method is particularly attractive as it can maintain patency of distal branches by retrograde flow from the distal end of the VA. Unfortunately, with proximal occlusion the aneurysm is still open to retrograde flow from the contralateral VA and perhaps the basilar artery if there were patent PCOMs, therefore maintaining the dissecting aneurysm’s ability to progress or bleed.[
Parent artery trapping
To more definitively control the dissecting aneurysm, the VA can be occluded both proximal and distal to the aneurysm (thereby completely excluding it from the circulation). For cases in which the origin of PICA is involved or nearby the aneurysm, this procedure would also sacrifice PICA with the compromise of its territory in the absence of robust collateral flow. Another less frequently discussed risk is the loss of the VA. The nondominant VA is usually considered safe to occlude, and even the dominant VA is frequently occluded, particularly if a large PCOM is present. However, following occlusion, the posterior circulation would almost entirely depend on the contralateral VA, which may not be sufficient or may suffer from contralateral disease involvement or resulting vasospasm.
Interestingly, this procedure has been performed in some cases without ischemic complications.[
Surprisingly, in our review of 24 cases treated with parent artery trapping, only one asymptomatic infarction in the PICA territory was documented. Incomplete occlusion was found in two cases. This relative success with parent artery trapping may result from the careful selection of patients who may tolerate obliteration of PICA. While the sacrifice of PICA is possible in some cases without neurological sequelae, particularly in cases with strong collateral supply to the PICA territory, it remains difficult to predict which patients may not tolerate this procedure.
Bypass
Trapping of an aneurysm involving PICA usually necessitates interrupting the antegrade flow through the branching vessel; however, multiple surgical approaches are available to revascularize PICA. PICA-PICA bypass, for example, can be performed in an elegant procedure that takes advantage of the anatomical proximity and similarity in the caliber of the two vessels.[
Trapping of the aneurysm and surgical revascularization of PICA can provide immediate, definitive treatment while still preserving PICA. Among 19 cases that were reviewed, two episodes of graft occlusion were reported (one asymptomatic and one causing symptomatic cerebellar infarction). Three other instances of infarction were also noted that may not have been related to the bypass procedure. Indeed, as this treatment is sometimes reserved for patients with few other options, the population may be over-represented with patients with more complicated presentations.
While bypass is very useful as a definitive treatment with a lower risk of rebleeding compared to endovascular methods, these procedures are technically challenging and require the patient to withstand surgery and its accompanying risks.[
Traditional stents
In contrast to the deconstructive methods, stenting provides a reconstructive method that heals the aneurysm while maintaining patency of the parent artery. Stents may also support coil placement within the aneurysm that would otherwise be precluded by the lack of a distinct aneurysmal neck. Modifications to these procedures include stent placement from the contralateral VA to PICA, to maintain patency of retrograde flow; stent-assisted coiling with the distal end of the stent within PICA, to prevent occlusion of the PICA origin;[
Risks to conventional stenting include insufficient redirection of flow away from the aneurysmal dilatation, leading to rebleeding, or aneurysmal progression.[
In the 12 patients reviewed that were treated with stent alone, aneurysm obliteration was incomplete in 8 (67%) by angiography, with one episode among these of rebleeding and death. The patient population may also have been biased toward relatively healthy patients. In the 38 patients treated with stent-assisted coiling, aneurysm obliteration was incomplete in 7 (18%) with four episodes of rebleeding and death and two episodes of cerebellar infarction. Curiously, the lack of aneurysmal obliteration did not always correspond to rebleeding; in one patient with complete obliteration, there was rebleeding, while many patients with incomplete obliteration did not experience any complications even after lengthy follow-up. Nonetheless, the high rebleeding rate suggests that stent placement is suboptimal for control of VADA.
Flow-diverting stents
The use of multiple overlapping stents offered high wall coverage and low porosity, thereby reducing blood flow to an aneurysmal dilatation while maintaining patency of the parent artery. Now, specialized stents have been designed to achieve this “flow-diverting” effect with a single device. While typical stents have wall coverage of 9–12%, flow-diverting stents may achieve wall coverage of 30–35%.[
A major advantage of flow-diverting stents derives from the ability to occlude flow to the aneurysm while preserving parent artery blood flow, regardless of the fusiform shape. Furthermore, the flow-diverting stent may maintain trans- stent perfusion of branching vessels, for example, PICA,[
Literature of flow-diverting stents, including in the posterior circulation, has accumulated only recently, and conclusions are only now starting to solidify. Among the reviewed studies, there were 30 that reported on the use of multiple overlapping stents, with only one instance of rebleeding. In addition, there were 10 cases treated using flow-diverting stents, with complications in two (stent thrombosis and occlusion, and the intraparenchymal hemorrhage in this case). Between both groups, the risk of rebleeding was only 1 in 40, and PICA was patent in all cases. However, the immediate occlusion rate was poor. These data suggest that, even though the flow-diverting stent is associated with variable degrees of angiographic improvement in the acute stage, the risk of rebleeding from the dissecting aneurysm is relatively low.
Other methods
Aside from surgical trapping, other surgical approaches are occasionally used for the treatment of VADA. Surgical aneurysm clipping offers an attractive option in saccular aneurysms, providing the most physiologic restoration of normal arterial flow with broad applicability to aneurysms at the VA-PICA junction.[
Treatment strategy
The treatment of VADA continues to improve with new techniques, most notably the development of flow-diverting stents. However, the approach to VADA remains multifaceted and requires consideration of many variables including PICA involvement, rupture, angiographic appearance, and evidence of progression, collateral flow and VA dominance, and other patient and operator factors. The number of potential treatment options parallels these considerations. In a series of 190 patients with VA aneurysms (150 saccular and 42 fusiform), Lehto et al. described more than 20 different treatment methods used at a single institution.[
Here, we reviewed literature for cases of VADA that involved PICA to clarify some of these points. Our study is limited by its design; as a retrospective literature review based on case series without standardized methods or inclusion criteria, the possibility for ascertainment bias, publication bias, differences in technique and skill, variable reporting standards, and other confounders inevitably confound the results. Indeed, as this is a rare entity, most of the reviewed literature consists of case series and the level of evidence is rather low. In the treatment of an individual patient, variables that cannot be controlled also factor in the response to treatment. However, the data presented point toward a general strategy for the management of this entity.
Due to the high risk of rebleeding leading to significant morbidity or mortality, the treatment of ruptured VADA must first focus on securing the aneurysm. Given the evidence presented in literature, we believe that parent artery trapping remains the most effective treatment in ruptured intracranial VADA, given sufficient distance of the aneurysm from PICA or other VA branches. Parent artery trapping has demonstrated a lower rate of rebleeding compared to proximal occlusion. The rate of immediate angiographic occlusion is also higher compared to the flow-diverting stent, which theoretically should yield better control of the vessel in the acute stage in which rebleeding is most likely. Furthermore, parent artery trapping obviates the risk of thromboembolic events from stent hardware or hemorrhage from dual antiplatelet treatment or anticoagulation. The disadvantage of trapping is the removal of one VA from the total flow of the posterior circulation. Two-thirds of patients have an incomplete circle of Willis. Thus, in view of the risk of compromise to the posterior circulation it would be important to know the total collateral circulation to the posterior circulation. Absence of one VA or qualitatively poor contribution from the anterior circulation through the PCOMs would be important factors in the selection of the treatment. The common belief that the patient can lose one VA if the other is patent is not backed by hard evidence that this assumption is true.[
In ruptured VADA with PICA involvement, the priority remains securing the aneurysm to prevent rebleeding and death. However, due to the risks of medullary infarction (which can range from asymptomatic to life threatening), flow-diverting stents should be considered first-line treatment. As an example, in our case, the patient appeared to have poor collateral flow to the distribution of the involved PICA and an incomplete circle of Willis, thereby disfavoring parent artery trapping. Flow-diverting stent placement was performed, with excellent immediate angiographic outcome. Published outcomes are excellent and the devices maintain patency of PICA in most cases. Care should be taken with monitoring of platelet function and special consideration for intracranial operations, for example, EVD or VP shunt placement, due to antiplatelet therapy.
In some cases, especially those with strong collaterals, the sacrifice of PICA along with the parent artery in a trapping procedure would provide more definitive treatment and should be considered. Unfortunately, the presence of collaterals and the importance of PICA are difficult to assess quantitatively. Surgical treatment with bypass methods may be considered in cases where the anatomy does not lend itself to the previously mentioned endovascular approaches and in which the patient has an acceptable risk of surgical complications.
Despite the published success with flow-diverting stents, the total experience with this treatment is still in its infancy and the complications must be better understood. The FDA- approved indications for Pipeline stenting in the United States have recently expanded to include intracranial aneurysms of the internal carotid artery (ICA) from the petrous ICA to the carotid terminus; flow diversion of the posterior circulation is, therefore, an off-label use. In addition, the optimal antiplatelet treatment strategy to prevent thromboembolic complications has not been determined. The most common regimen is preprocedural and postprocedural dual antiplatelet therapy with aspirin and clopidogrel, with discontinuation of clopidogrel and reduction of aspirin dose over time, though heterogeneity exists in the dosages used, duration of therapy, and use of platelet function testing.[
CONCLUSION
These guidelines provide a framework for considering the treatment options for ruptured, intracranial VADA, particularly involving PICA. However, uncertainty continues to surround the treatment of this precarious condition. The collateral flow of PICA and the competency of the contralateral VA are difficult to quantify precisely, and therefore the operative risk of ischemia to these important arterial distributions can only be approximated. Furthermore, the success of treatment (as measured by immediate angiography) does not seem to be highly correlated with the durability of outcome, in instances of using conventional and flow-diverting stents. Efforts in identifying predictors of clinical outcome and treatment complications will be important to determine the optimal treatment option for individual patients.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understands that his name and initials will not be published and due efforts will be made to conceal his identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Aihara M, Naito I, Shimizu T, Matsumoto M, Asakura K, Miyamoto N. Predictive factors of medullary infarction after endovascular internal trapping using coils for vertebral artery dissecting aneurysms. J Neurosurg. 2017. 129: 1-7
2. Al-khayat H, Al-Khayat H, Beshay J, Manner D, White J. Vertebral artery-posteroinferior cerebellar artery aneurysms: Clinical and lower cranial nerve outcomes in 52 patients. Neurosurgery. 2005. 56: 2-10
3. Aoki N, Sakai T. Rebleeding from intracranial dissecting aneurysm in the vertebral artery. Stroke. 1990. 21: 1628-31
4. Atallah E, Saad H, Bekelis K, Chalouhi N, Tjoumakaris S, Hasan D. The use of alternatives to clopidogrel in flow-diversion treatment with the pipeline embolization device. J Neurosurg. 2018. 129: 1130-5
5. Ausman JI, Diaz FG, Mullan S, Gehring R, Sadasivan B, Dujovny M. Posterior inferior to posterior inferior cerebellar artery anastomosis combined with trapping for vertebral artery aneurysm. Case report. J Neurosurg. 1990. 73: 462-5
6. Ausman JI, Liebeskind DS, Gonzalez N, Saver J, Martin N, Villablanca JP. A review of the diagnosis and management of vertebral basilar (posterior) circulation disease. Surg Neurol Int. 2018. 9: 106-
7. Bender MT, Colby GP, Jiang B, Lin LM, Campos JK, Xu R. Flow diversion of posterior circulation cerebral aneurysms: A single-institution series of 59 cases. Neurosurgery. 2019. 84: 206-16
8. Bender MT, Zarrin DA, Campos JK, Jiang B, Chandra A, Vo CD.editors. Precision of verifyNow P2Y12 assessment of clopidogrel response in patients undergoing cerebral aneurysm flow diversion. Neurosurgery. 2018. p.
9. Benndorf G, Herbon U, Sollmann WP, Campi A. Treatment of a ruptured dissecting vertebral artery aneurysm with double stent placement: Case report. AJNR Am J Neuroradiol. 2001. 22: 1844-8
10. Berger MS, Wilson CB. Intracranial dissecting aneurysms of the posterior circulation. Report of six cases and review of the literature. J Neurosurg. 1984. 61: 882-94
11. Czabanka M, Ali M, Schmiedek P, Vajkoczy P, Lawton MT. Vertebral artery-posterior inferior cerebellar artery bypass using a radial artery graft for hemorrhagic dissecting vertebral artery aneurysms: Surgical technique and report of 2 cases. J Neurosurg. 2011. 114: 1074-9
12. Fiorella D, Woo HH, Albuquerque FC, Nelson PK. Definitive reconstruction of circumferential, fusiform intracranial aneurysms with the pipeline embolization device. Neurosurgery. 2008. 62: 1115-20
13. Friedman AH, Drake CG. Subarachnoid hemorrhage from intracranial dissecting aneurysm. J Neurosurg. 1984. 60: 325-34
14. Frontera JA, Claassen J, Schmidt JM, Wartenberg KE, Temes R, Connolly ES. Prediction of symptomatic vasospasm after subarachnoid hemorrhage: The modified fisher scale. Neurosurgery. 2006. 59: 21-7
15. Gupta R, Moore JM, Griessenauer CJ, Adeeb N, Patel AS, Youn R. Assessment of dual-antiplatelet regimen for pipeline embolization device placement: A survey of major academic neurovascular centers in the united states. World Neurosurg. 2016. 96: 285-92
16. Hashimoto M, Johkura K, Ichikawa T, Kojima A, Nishimura S, Shinonaga M. Conservative treatment of ruptured vertebrobasilar dissecting aneurysm. Neurol Sci. 2008. 29: 241-4
17. Kai Y, Nishi T, Watanabe M, Morioka M, Hirano T, Yano S. Strategy for treating unruptured vertebral artery dissecting aneurysms. Neurosurgery. 2011. 69: 1085-91
18. Kaku Y, Yoshimura S, Yamakawa H, Sakai N. Failure of stent-assisted endovascular treatment for ruptured dissecting aneurysms of the basilar artery. Neuroradiology. 2003. 45: 22-6
19. Kallmes DF, Ding YH, Dai D, Kadirvel R, Lewis DA, Cloft HJ. A new endoluminal, flow-disrupting device for treatment of saccular aneurysms. Stroke. 2007. 38: 2346-52
20. Kim BM, Shin YS, Kim SH, Suh SH, Ihn YK, Kim DI. Incidence and risk factors of recurrence after endovascular treatment of intracranial vertebrobasilar dissecting aneurysms. Stroke. 2011. 42: 2425-30
21. Kitanaka C, Sasaki T, Eguchi T, Teraoka A, Nakane M, Hoya K. Intracranial vertebral artery dissections: Clinical, radiological features, and surgical considerations. Neurosurgery. 1994. 34: 620-6
22. Kitanaka C, Morimoto T, Sasaki T, Takakura K. Rebleeding from vertebral artery dissection after proximal clipping. Case report. J Neurosurg. 1992. 77: 466-8
23. Kobayashi S, Karasudani H, Koguchi Y, Tsuru K, Wada M, Miyata A. Endovascular treatment for ruptured VA dissecting aneurysm involving the origin of PICA. Interv Neuroradiol. 2004. 10: 173-9
24. Lehto H, Kivisaari R, Niemelä M, Dashti R, Elsharkawy A, Harati A. Seventy aneurysms of the posterior inferior cerebellar artery: Anatomical features and value of computed tomography angiography in microneurosurgery. World Neurosurg. 2014. 82: 1106-12
25. Lehto H, Niemelä M, Kivisaari R, Laakso A, Jahromi BR, Hijazy F. Intracranial vertebral artery aneurysms: Clinical features and outcome of 190 patients. World Neurosurg. 2015. 84: 380-9
26. Levitt MR, Park MS, Albuquerque FC, Moon K, Kalani MY, McDougall CG. Posterior inferior cerebellar artery patency after flow-diverting stent treatment. AJNR Am J Neuroradiol. 2016. 37: 487-9
27. Madaelil TP, Wallace AN, Chatterjee AN, Zipfel GJ, Dacey RG, Cross DT. Endovascular parent vessel sacrifice in ruptured dissecting vertebral and posterior inferior cerebellar artery aneurysms: Clinical outcomes and review of the literature. J Neurointerv Surg. 2016. 8: 796-801
28. Mizutani T, Aruga T, Kirino T, Miki Y, Saito I, Tsuchida T. Recurrent subarachnoid hemorrhage from untreated ruptured vertebrobasilar dissecting aneurysms. Neurosurgery. 1995. 36: 905-11
29. Park MS, Albuquerque FC, Nanaszko M, Sanborn MR, Moon K, Abla AA. Critical assessment of complications associated with use of the pipeline embolization device. J Neurointerv Surg. 2015. 7: 652-9
30. Peluso JP, van Rooij WJ, Sluzewski M, Beute GN, Majoie CB. Endovascular treatment of symptomatic intradural vertebral dissecting aneurysms. AJNR Am J Neuroradiol. 2008. 29: 102-6
31. Rabinov JD, Hellinger FR, Morris PP, Ogilvy CS, Putman CM. Endovascular management of vertebrobasilar dissecting aneurysms. AJNR Am J Neuroradiol. 2003. 24: 1421-8
32. Ro A, Kageyama N. Pathomorphometry of ruptured intracranial vertebral arterial dissection: Adventitial rupture, dilated lesion, intimal tear, and medial defect. J Neurosurg. 2013. 119: 221-7
33. Sanai N, Tarapore P, Lee AC, Lawton MT. The current role of microsurgery for posterior circulation aneurysms: A selective approach in the endovascular era. Neurosurgery. 2008. 62: 1236-49
34. Sasaki O, Ogawa H, Koike T, Koizumi T, Tanaka R. A clinicopathological study of dissecting aneurysms of the intracranial vertebral artery. J Neurosurg. 1991. 75: 874-82
35. Sato T, Sasaki T, Suzuki K, Matsumoto M, Kodama N, Hiraiwa K. Histological study of the normal vertebral artery-etiology of dissecting aneurysms. Neurol Med Chir (Tokyo). 2004. 44: 629-35
36. Satow T, Ishii D, Iihara K, Sakai N, JR-NET study group. Endovascular treatment for ruptured vertebral artery dissecting aneurysms: Results from Japanese registry of neuroendovascular therapy (JR-NET) 1 and 2. Neurol Med Chir (Tokyo). 2014. 54: 98-106
37. Schömig A, Neumann FJ, Kastrati A, Schühlen H, Blasini R, Hadamitzky M. A randomized comparison of antiplatelet and anticoagulant therapy after the placement of coronary-artery stents. N Engl J Med. 1996. 334: 1084-9
38. Steinberg GK, Drake CG, Peerless SJ. Deliberate basilar or vertebral artery occlusion in the treatment of intracranial aneurysms. Immediate results and long-term outcome in 201 patients. J Neurosurg. 1993. 79: 161-73
39. Su W, Gou S, Ni S, Li G, Liu Y, Zhu S. Management of ruptured and unruptured intracranial vertebral artery dissecting aneurysms. J Clin Neurosci. 2011. 18: 1639-44
40. Takagi T, Takayasu M, Suzuki Y, Yoshida J. Prediction of rebleeding from angiographic features in vertebral artery dissecting aneurysms. Neurosurg Rev. 2007. 30: 32-8
41. Tan LA, Keigher KM, Munich SA, Moftakhar R, Lopes DK. Thromboembolic complications with pipeline embolization device placement: Impact of procedure time, number of stents and pre-procedure P2Y12 reaction unit (PRU) value. J Neurointerv Surg. 2015. 7: 217-21
42. Taylor LI, Dickerson JC, Dambrino RJ, Kalani MY, Taussky P, Washington CW. Platelet testing in flow diversion: A review of the evidence. Neurosurg Focus. 2017. 42: E5-
43. Wu Z, Lv X, Yang X, He H. Ruptured vertebro-inferoposterior cerebellar artery dissecting aneurysm treated with the neuroform stent deployment and vertebral artery occlusion. Eur J Radiol Extra. 2009. 70: e100-3
44. Yoshimoto Y, Wakai S. Unruptured intracranial vertebral artery dissection. Clinical course and serial radiographic imagings. Stroke. 1997. 28: 370-4
45. Zhao KJ, Zhao R, Huang QH, Xu Y, Hong B, Fang YB. The interaction between stent(s) implantation, PICA involvement, and immediate occlusion degree affect symptomatic intracranial spontaneous vertebral artery dissection aneurysm (sis-VADA) recurrence after reconstructive treatment with stent(s)-assisted coiling. Eur Radiol. 2014. 24: 2088-96