- Department of Neurosurgery, Fukuoka Neurosurgical Hospital, Fukuoka, Japan.
Correspondence Address:
Shinichiro Yoshida, Department of Neurosurgery, Fukuoka Neurosurgical Hospital, Fukuoka, Japan.
DOI:10.25259/SNI_961_2022
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Shinichiro Yoshida, Kaisei Kamatani, Kousei Maruyama, Yoshiaki Hama, Noriaki Tashiro, Fumihiro Hiraoka, Shigetoshi Yano, Hiroshi Aikawa, Yoshinori Go, Kiyoshi Kazekawa. Treatment strategy for giant thrombosed aneurysm of the basilar artery with associated obstructive hydrocephalus. 20-Jan-2023;14:23
How to cite this URL: Shinichiro Yoshida, Kaisei Kamatani, Kousei Maruyama, Yoshiaki Hama, Noriaki Tashiro, Fumihiro Hiraoka, Shigetoshi Yano, Hiroshi Aikawa, Yoshinori Go, Kiyoshi Kazekawa. Treatment strategy for giant thrombosed aneurysm of the basilar artery with associated obstructive hydrocephalus. 20-Jan-2023;14:23. Available from: https://surgicalneurologyint.com/surgicalint-articles/12108/
Abstract
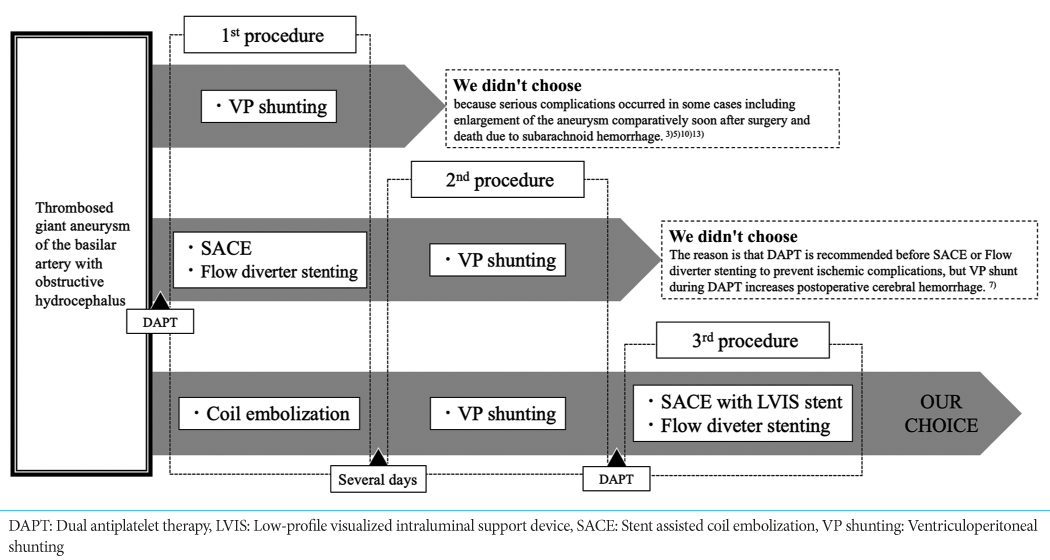
Background: There is no established adequate treatment for thrombosed aneurysm of the basilar artery with obstructive hydrocephalus. We conducted coil embolization and peritoneal shunting followed by placement of a stent expected to exert flow diversion (FD) effects to treat 2 patients with giant thrombosed aneurysms of the basilar artery with associated obstructive hydrocephalus, with good results.
Methods: From April 2019 to March 2021, consecutive two cases of symptomatic hydrocephalus due to giant thrombosed aneurysms in the posterior cranial fossa at our hospital were treated. At first, coil embolization was performed to prevent aneurysm rupture. After coil embolization, ventriculoperitoneal shunting was performed. Finally, stent-assisted coil embolization was performed with flow re-direction endoluminal device (FRED) or low-profile visualized intraluminal support device (LVIS) stent.
Results: Both patients were discharged after recovering well, with no postoperative hemorrhagic or ischemic complications.
Conclusion: Staged surgery using a FRED for flow diverter or an LVIS stent expected to have FD effects may offer an effective treatment option.
Keywords: Flow re-direction endoluminal device, Hydrocephalus, Low-profile visualized intraluminal support device, Neuroendovascular, Thrombosed aneurysm
INTRODUCTION
The prognosis for giant thrombosed aneurysms in the posterior cranial fossa is extremely poor, and the mortality rate is very high if the aneurysm is left untreated.[
We conducted coil embolization and VP shunting, followed by placement of a low-profile visualized intraluminal support device (LVIS stent) (MicroVention, Tustin, CA, USA) or flow re-direction endoluminal device (FRED) (MicroVention, Tustin, CA, USA) for flow diverter in expectation of flow diversion (FD) effects, to treat two patients with giant thrombosed aneurysm and associated obstructive hydrocephalus. We report these staged procedures with discussion of the relevant literature.
MATERIALS AND METHODS
From April 2019 to March 2021, consecutive two cases of symptomatic hydrocephalus due to giant thrombosed aneurysms in the posterior cranial fossa at our hospital were treated.
At first coil embolization was performed to prevent aneurysm rupture. Single antiplatelet therapy (SAPT) was started before the first coil embolization to prevent intraoperative and postoperative ischemic complications. The right femoral artery was punctured and a 6-Fr short sheath was inserted. A 6-Fr Roadmaster guiding catheter (Goodman, Aichi, Japan) was guided into the left vertebral artery. An Echelon 10 microcatheter was, then, inserted and guided into the aneurysm with a CHIKAI 0.014-inch guidewire (Asahi Intecc, Aichi, Japan). A second Echelon 10 microcatheter was guided into the aneurysm with a CHIKAI 0.014-inch guidewire. Due to the large size of the aneurysm, the double-catheter technique was employed for embolization. After coil embolization, VP shunting was performed. The Codman CERTAS Plus (Integra LifeSciences, Princeton, New Jersey, USA) was used as the shunt valve.
Dual antiplatelet therapy (DAPT) was started after shunt placement and continued for over 10 days until second operation to prevent subsequent intraoperative and postoperative ischemic complications.
Stent-assisted coil embolization (SACE) was performed with FRED or LVIS stent. The right femoral artery was punctured and an 8-Fr short sheath was inserted. A Roadmaster guiding catheter was guided into the vertebral artery to the level of the 2nd cervical vertebra. A Headway 27 microcatheter (MicroVention, Tustin, CA, USA) or Headway 21 microcatheter (MicroVention, Tustin, CA, USA) was inserted with a CHIKAI 0.014-inch guidewire to the basilar artery, from where FRED or LVIS stent was deployed. When using LVIS stent, an Echelon 10 microcatheter was guided into the residual aneurysm with a CHIKAI 0.014-inch guidewire and used for coil embolization.
Finally, DAPT was continued over 6 months to prevent postoperative ischemic complications.
RESULTS
All surgical procedure was completed [
Figure 1:
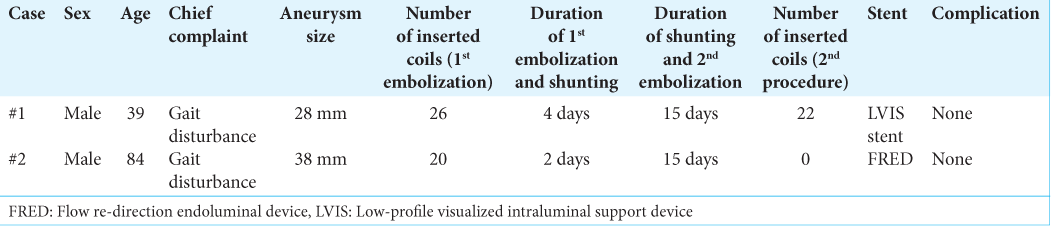
Preoperative images of case# 1 and case# 2. (a) Axial T2-weighted MRI. (b) Axial and coronal CT. Ventricular enlargement with Evans index>0.3, sharpening of the callosal angle, and disproportionately enlarged subarachnoid space hydrocephalus were observed. (c) Frontal digital subtraction angiography (DSA). (d) Lateral DSA.
Figure 3:
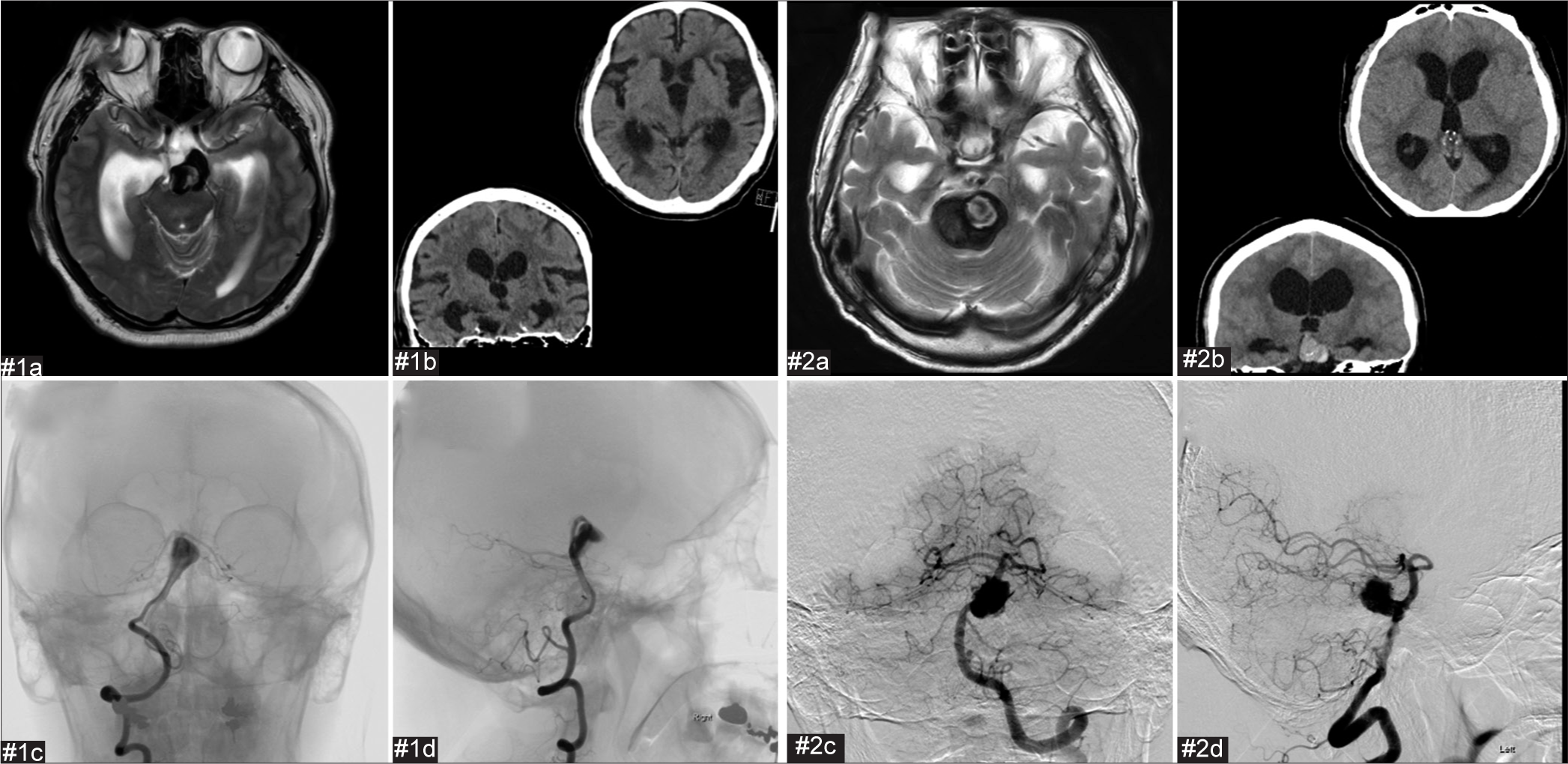
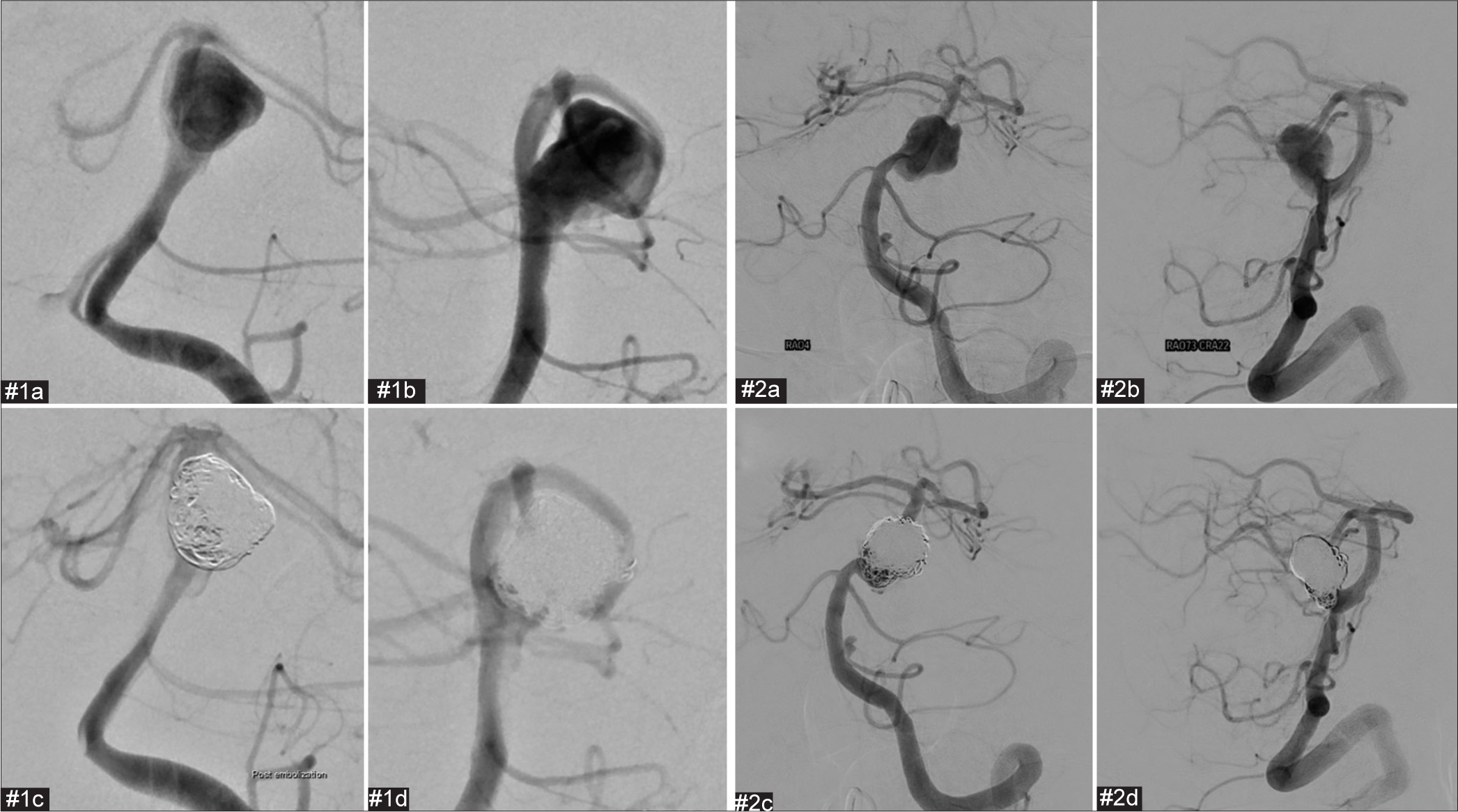
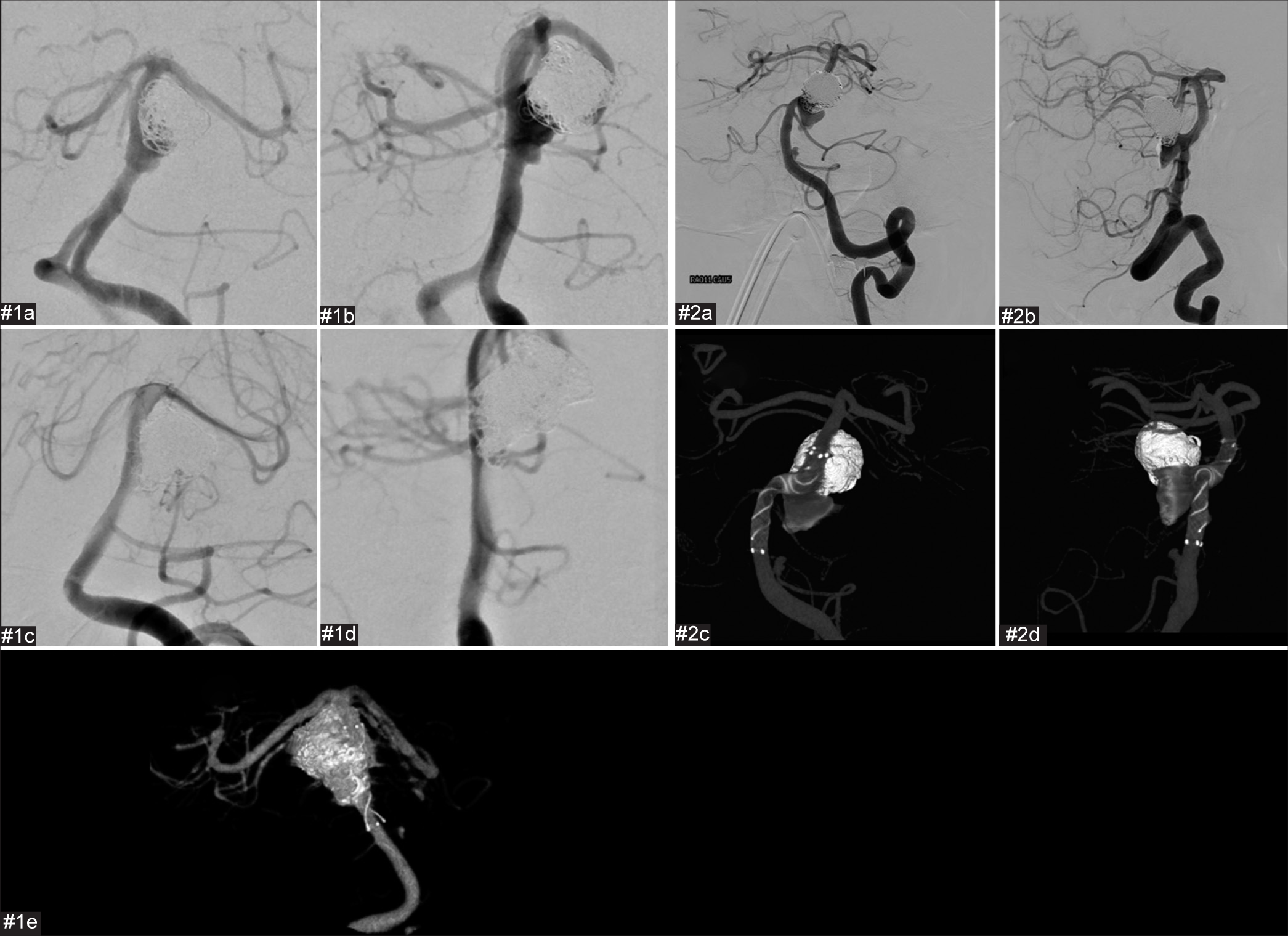
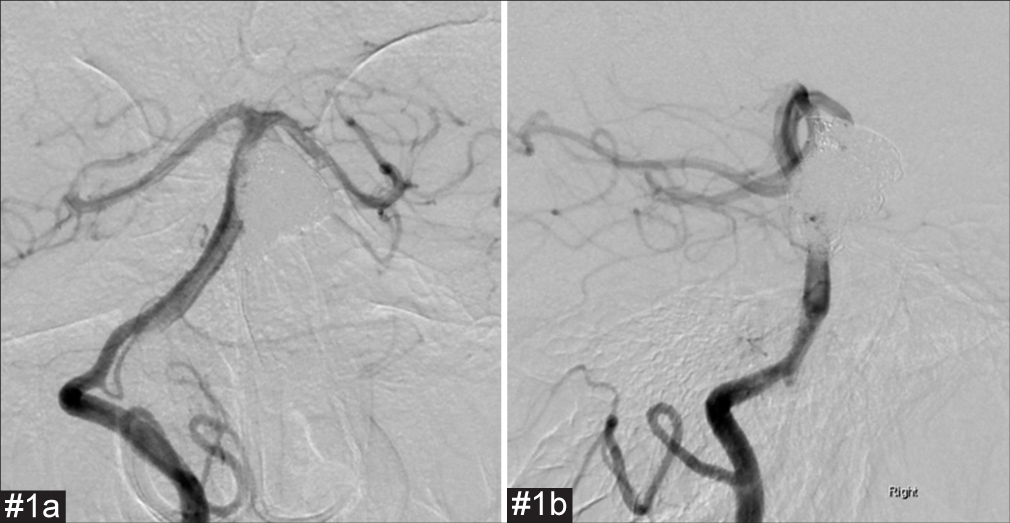
Postoperative and preoperative images of the second coil embolization and stent employment for cases #1 and #2. The proximal part of the aneurysm has enlarged compared to immediately after the initial procedure. We considered that the aneurysm had enlarged as a result of coil embolization of the thrombosed aneurysm. #1(a) Preoperative frontal digital subtraction angiography (DSA). #1(b) Preoperative lateral DSA. #1(c) Postoperative frontal DSA. #1(d) Postoperative lateral DSA. #1(e) Cone-beam computed tomography (CT). #2(a) Preoperative frontal DSA. #2(b) Preoperative lateral DSA. #2(c) Postoperative frontal image in cone-beam CT. #2(d) Postoperative lateral image in cone-beam CT.
DISCUSSION
Giant thrombotic aneurysms in the posterior cranial fossa that causes a mass effect have an extremely poor prognosis, including the development of neurological abnormalities due to brainstem compression. If left untreated, the mortality rate reportedly exceeds 50% within 2–3 years.[
SAPT was started before the first coil embolization, and DAPT before the second cerebral endovascular treatment was administered over 10 days after the shunt operation. Consensus regarding the method of duration of DAPT before cerebral endovascular treatment has yet to be reached. Because DAPT should be started 5–7 days before using a flow diverter stent[
In both patients, cerebral angiography 2 weeks after coil embolization revealed enlargement of the aneurysm due to coil compaction. We subsequently placed either a flow diverter or an LVIS stent expected to have an FD effect in addition to further coil embolization, with good results. The metal coverage rate of the LVIS stent is 18–29%,[
CONCLUSION
We conducted coil embolization before VP shunting, followed shortly after by placement of a FRED for flow diverter or LVIS stent expected to have FD effects, to treat two patients with giant thrombosed aneurysm with associated obstructive hydrocephalus, without the occurrence of any hemorrhagic or ischemic complications. Staged surgery including placement of a FRED for flow diverter or LVIS stent expected to have FD effects may represent an effective treatment option.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Borrie MJ, Campbell AJ, Cradoc-Davies TH. Obstructive hydrocephalus secondary to giant cerebral aneurysm in the elderly: Two case reports of functional improvement after ventricular shunting. J Am Geriatr. 1985. 33: 210-2
2. Da Ros V, Caroff J, Rouchaud A, Mihalea C, Ikka L, Moret J. Large basilar apex anerurysm treated with flow-diverter stents. AJNR Am J Neuroradiol. 2017. 38: 1156-62
3. Drake CG. Giant intracranial aneurysms: experience with surgical treat-ment in 174 patients. Clin Neurosurg. 1979. 26: 12-95
4. Feng Z, Fang Y, Xu Y, Hong B, Zhao W, Liu J. The safety and efficacy of low profile visualized intraluminal support (LVIS) atents in assisting coil embolization of intracranial saccular aneurysms: A single center experience. J Neurointerv Surg. 2016. 8: 1192-6
5. Goetz C, Seifert V, Haubitz B. The foramen of Monro-blockage caused by a giant aneurysm of the basilar artery. A case report and review of the literature. Neurochirurgia (Stuttg). 1990. 33: 122-6
6. Hashimoto N, Hanada H. The fate of untreated symptomatic cerebral aneurysms: Analysis of 26 patients with clinical course of more than five years. Surg Neurol. 1982. 18: 21-6
7. Hudson JS, Nagamaha Y, Nakagawa D, Starke RM, Dlouhy BJ, Torner JC. Hemorrhage associated with ventriculoperitoneal shunt placement in aneurysmal subarachnoid hemorrhage patients on a regimen of dual antiplatelet therapy: A retrospective analysis. J Neurosurg. 2018. 129: 916-21
8. Kano T. Unruptured thrombosed giant aneurysm: Strategy for treatment. Surg Cereb Stroke. 2003. 31: 344-8
9. Kim KS, Fraser JF, Grupke S, Cook AM. Management of antiplatelet therapy in patients undergoing neuroendovascular procedures. J Neurosurg. 2018. 129: 890-905
10. Kim MS, Oh CW, Han DH. Growth of basilar artery aneurysm after ventriculo-peritoneal shunt. J Clin Neurosci. 2002. 9: 696-702
11. Liu JK, Gottfried ON, Couldwell WT. Thrombosed basilar apex aneurysm presenting as a third ventricular mass and hydrocephalus. Acta Neurochir (Wien). 2005. 147: 413-7 discussion 417
12. Morley TP, Barr HW. Giant intracranial aneurysms: Diagnosis, course, and management. Clin Neurosurg. 1969. 16: 73-94
13. Soeur M, Brihaye J, Moerman C. Pseudo-tumoural aneurysm in the third ventricle: Report of three cases. Acta Neurochir. 1979. 45: 247-58
14. Tonetti DA, Jankowitz BT, Gross BA. Antiplatelet therapy in flow diversion. Neurosurgery. 2020. 86: 47-52
15. Tremmel M, Xiang J, Natarajan SK, Hopkins LN, Siddiqui AH, Levy EI. Alteration of intra-aneurysmal hemodynamics for flow diversion using enterprise and vision stents. World Neurosurg. 2010. 74: 306-15
16. Tsutsumi S, Kondo A, Abe Y, Yasumoto Y, Ito M. Basilar apex anerurysm manifesting as third ventricular mass and obstructive hydrocephalus. Neurol Med Chir (Tokyo). 2008. 48: 451-4
17. Watanabe A, Imamura K, Ishii R. Endosaccular aneurysm occlusion with Guglielimi detachable coils for obstructive hydrocephalus caused by a large basilar tip aneurysm. J Neurosurg. 1999. 91: 675-8