- Department of Neurosurgery and Stroke Center, Tenri Hospital, Tenri, Japan.
Correspondence Address:
Kampei Shimizu, Department of Neurosurgery and Stroke Center, Tenri Hospital, Tenri, Japan.
DOI:10.25259/SNI_848_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Masahiko Itani, Kampei Shimizu, Shoichi Tani, Motoaki Fujimoto, Hideki Ogata, Shota Yoshida, Yoshihito Hirata, Yoshinori Akiyama. True superficial temporal artery aneurysm: A case after extracranial-intracranial bypass surgery and a systematic review. 09-Dec-2022;13:573
How to cite this URL: Masahiko Itani, Kampei Shimizu, Shoichi Tani, Motoaki Fujimoto, Hideki Ogata, Shota Yoshida, Yoshihito Hirata, Yoshinori Akiyama. True superficial temporal artery aneurysm: A case after extracranial-intracranial bypass surgery and a systematic review. 09-Dec-2022;13:573. Available from: https://surgicalneurologyint.com/surgicalint-articles/12050/
Abstract
Background: Nontraumatic true superficial temporal artery aneurysm (STAA) is rare, and its characteristics and pathogenesis are unclear.
Methods: We report a case of STAA and performed a systematic review of PubMed, Scopus, and Web of Science using the keyword “superficial temporal artery aneurysm” to include studies on STAA reported through July 2022. We excluded studies on STAA associated with trauma, arterial dissection, infection, or vasculitis.
Results: A 63-year-old woman who underwent left superficial temporal artery (STA)-middle cerebral artery bypass surgery 8 years previously was diagnosed with an aneurysm located at the left STA. The blood flow volume estimated by ultrasonography was higher in the left STA than in the contralateral counterpart (114 mL/min vs. 32 mL/min). She underwent clipping surgery to prevent aneurysmal rupture without sequela. The lesion was diagnosed as a true aneurysm by histology. The systematic review identified 63 cases (including the present case) of nontraumatic true STAA. The median age of the patients was 57 (interquartile range [IQR]: 41–70) years. Most (90.5%) cases were detected as a palpable mass. Aneurysmal rupture occurred in only 1 (1.6%) case, despite the large size of aneurysms (median size: 13 [IQR: 8–20] mm) and the high frequency (33.3%) of aneurysmal growth during observation. Most (93.7%) patients underwent surgical resection of STAA without sequela.
Conclusion: Our findings suggest that the pathogenesis of true STAA is promoted by hemodynamic stress. The systematic review clarified patients’ and aneurysmal characteristics and treatment outcomes, providing further insight into the pathogenesis of nontraumatic true STAA.
Keywords: Bypass, Intracranial aneurysm, Segmental arterial mediolysis, Superficial temporal artery aneurysm, Systematic review
INTRODUCTION
Superficial temporal artery aneurysm (STAA) is an uncommon disease, and approximately 90% of STAAs are traumatic pseudoaneurysms.[
We report here a rare case of a true STAA that developed more than 2 years after superficial temporal artery (STA)-middle cerebral artery (MCA) bypass surgery. The findings in the present case suggest the potential pathogenesis leading to the development of true STAA and show a rare complication after STA-MCA bypass surgery. We also performed a systematic review of the literature to investigate the characteristics, treatment outcomes, and potential pathogenesis of nontraumatic true STAA.
MATERIALS AND METHODS
This study was conducted following the principles of the Declaration of Helsinki and its later amendments. The patient provided written informed consent for the treatment rendered. This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.[
Search strategy and selection criteria
We performed a systematic review of PubMed, Scopus, and Web of Science to identify eligible studies published between the date of the databases’ inception and July 2022 using the keyword “superficial temporal artery aneurysm.” We also searched the literature referenced by eligible studies for any possible missing studies. The present systematic review aimed to analyze the characteristics, treatment outcomes, and potential pathogenesis of nontraumatic true STAA. Therefore, the inclusion criteria were studies reporting STAA. The exclusion criteria were studies on STAA associated with trauma, arterial dissection, infection, or systemic inflammatory diseases such as vasculitis. Articles written in languages other than English or Japanese were translated using Google translate (
Data extraction
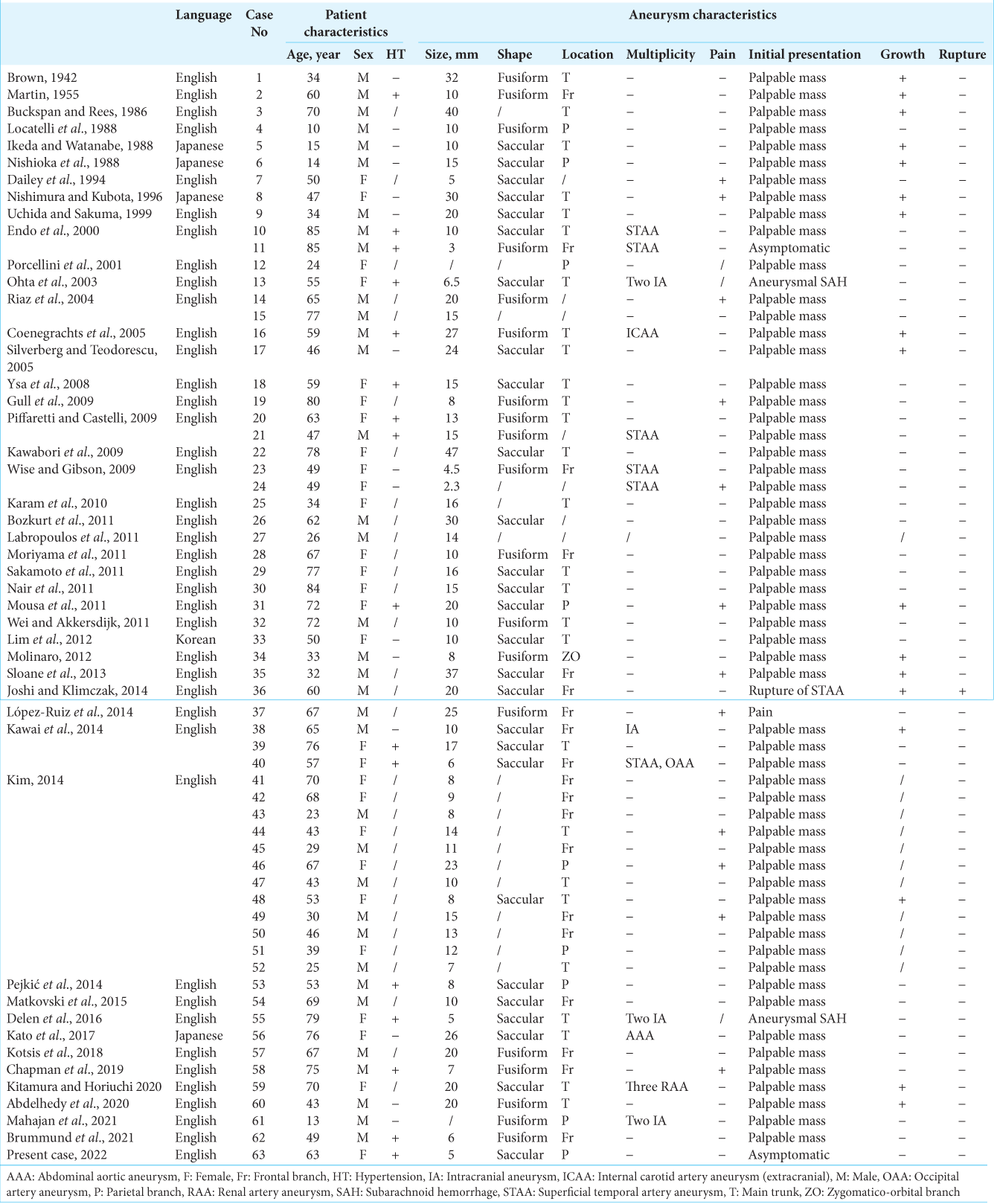
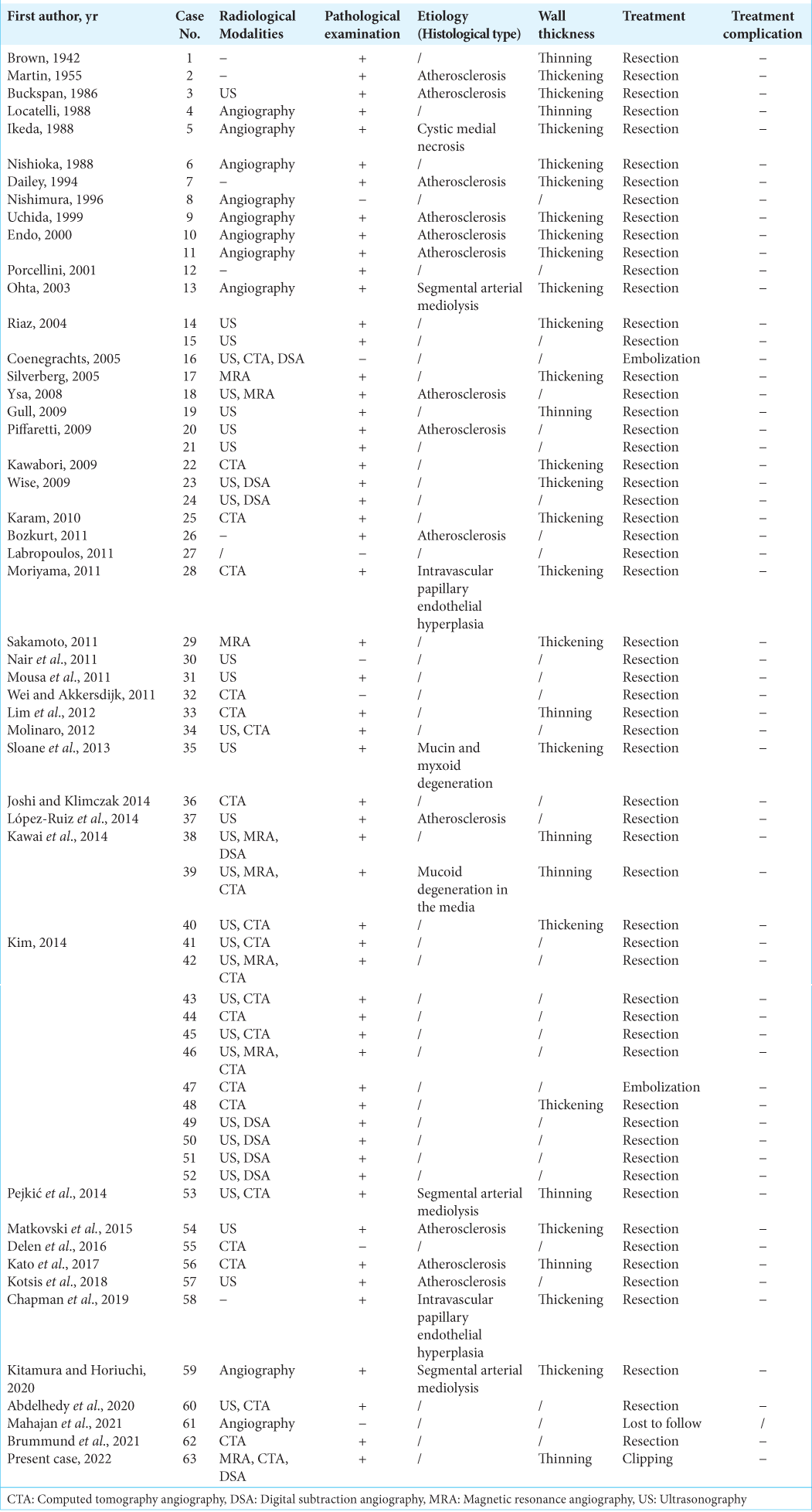
Two investigators (M.I. and K.S.) independently completed the database search. Any discrepancies between investigators were resolved by discussion. We extracted data from the included studies using a standardized form consisting of the following factors: patients’ characteristics (age, sex, and a history of hypertension); aneurysmal characteristics, such as size, shape (i.e., saccular or fusiform), location (e.g., the frontal branch of the STA), multiplicity, symptoms (e.g., tenderness), growth during observation, and rupture status; and factors regarding diagnosis or treatment, such as diagnostic modalities, histological findings regarding the etiology and wall thickness, treatment modalities, and treatment complications. In this study, multiplicity indicated if the patient had another aneurysm, such as an intracranial aneurysm (IA), visceral aneurysm (e.g., renal artery aneurysm), or aortic aneurysm [
The extracted data are shown as the median (interquartile range [IQR]) or frequency (i.e., %). To assess the pathogenesis of STAA, we compared the characteristics of STAA with those of IA by referencing the International Study of Unruptured IA Investigators performed in the U.S.A, Canada, and Europe,[
RESULTS
Case report
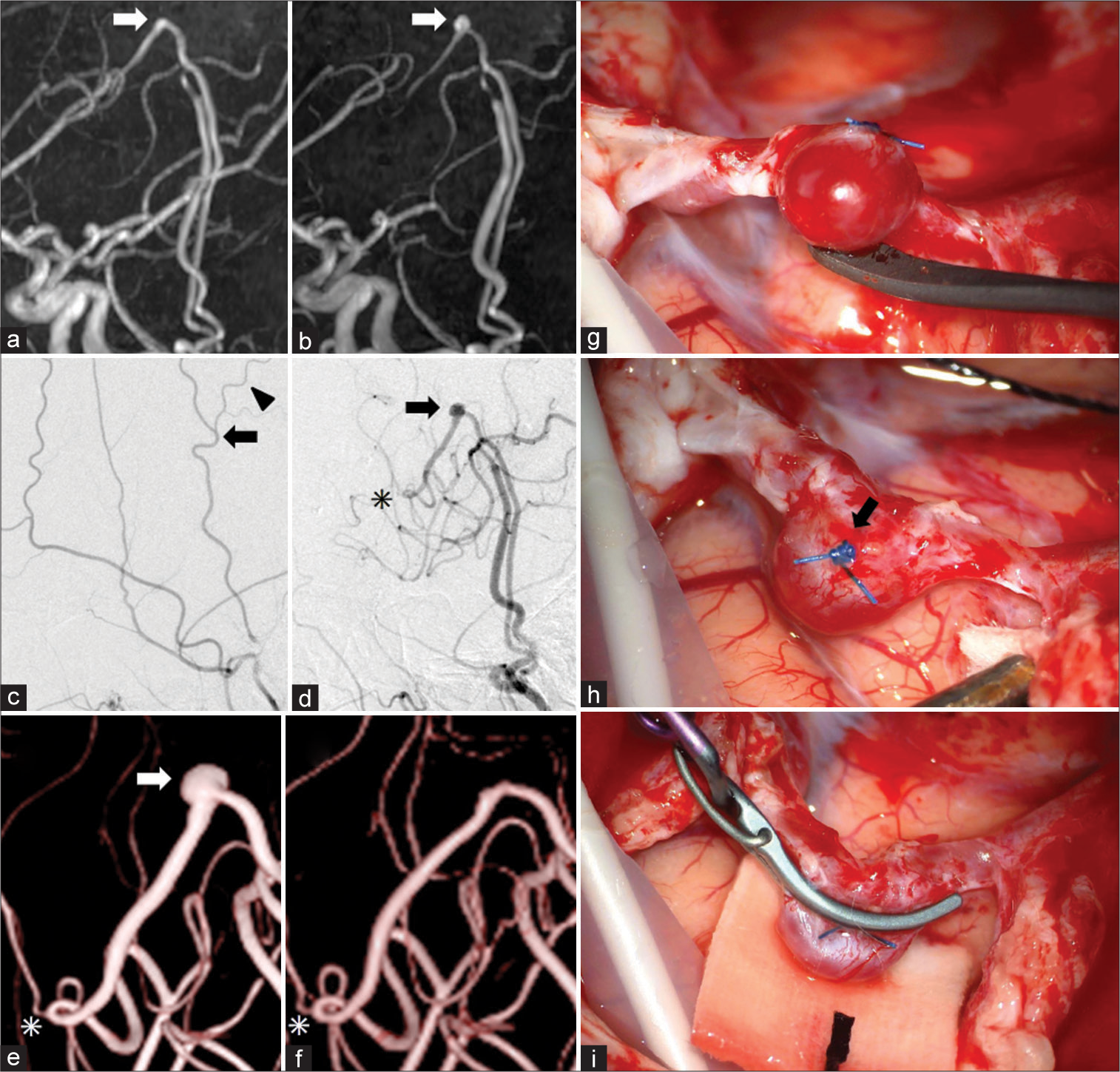
A 55-year-old woman with a medical history of hypertension and dyslipidemia visited our hospital complaining of weakness in the right upper extremity. A radiological examination showed cerebral infarction and hemodynamic compromise due to the left MCA stenosis. Therefore, she underwent revascularization by the left STA-MCA bypass. Two-year postoperative magnetic resonance angiography showed no aneurysm formation at the left STA [
Figure 1:
Radiological and intraoperative findings. (a and b) Two- (a) and 8-year (b) postoperative magnetic resonance angiography shows the development of a superficial temporal artery aneurysm (STAA) between 2 and 8 years after superficial temporal artery (STA)-middle cerebral artery bypass surgery. The STAA is localized at a sharp bend of the left STA (arrows in a and b) proximal to the anastomosis. (c and d) Preoperative (c) and 8-year postoperative (d) digital subtraction angiography show a de novo saccular STAA (5 mm in size) on the outer curvature of the left STA (arrows in c and d) and a postoperative increase in the diameter of the left STA. A small branch from the STA (arrowhead in c) was ligated using a 6-0 nylon suture and cut before the anastomosis. The site of the anastomosis is indicated by an asterisk in (d). (e and f) Three-dimensional rotational angiography before (e) and after (f) clipping surgery. The STAA is indicated by an arrow in (e), and the site of the anastomosis is indicated by asterisks in (e) and (f). (g-i) Intraoperative findings of the STAA. The appearance of the STAA resembles that of usual intracranial aneurysms. A 6-0 nylon suture (arrow in h) used to ligate the branch indicated by the arrowhead in (c) remains and was useful in identifying the precise location of the STAA.
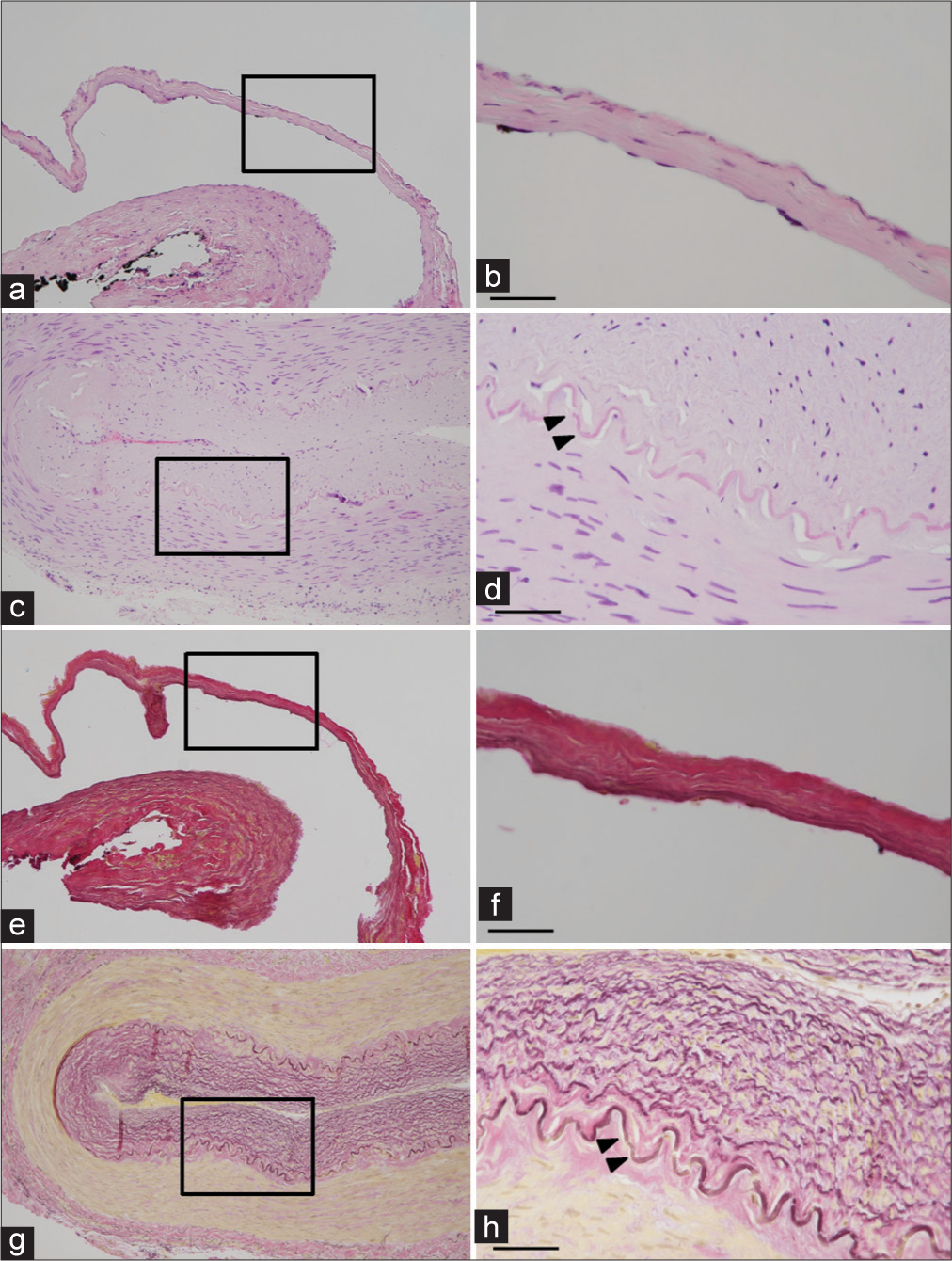
Figure 2:
Histopathological investigation of the superficial temporal artery aneurysm (STAA) and a control superficial temporal artery (STA) in a 79-year-old man. Hematoxylin and eosin (a-d) and Elastica van Gieson (e-h) staining of the STAA (a, b, e, and f) and the control STA (c, d, g, and h) are shown. The magnified views indicated by the squares in (a, c, e, and g) are shown in (b, d, f, and h), respectively. The internal elastic lamina in the control STA is indicated by arrowheads in (d and h). The internal elastic lamina was absent in the STAA. The aneurysmal wall was thin and hypocellular. Scale bar, 50 μm.
Search results
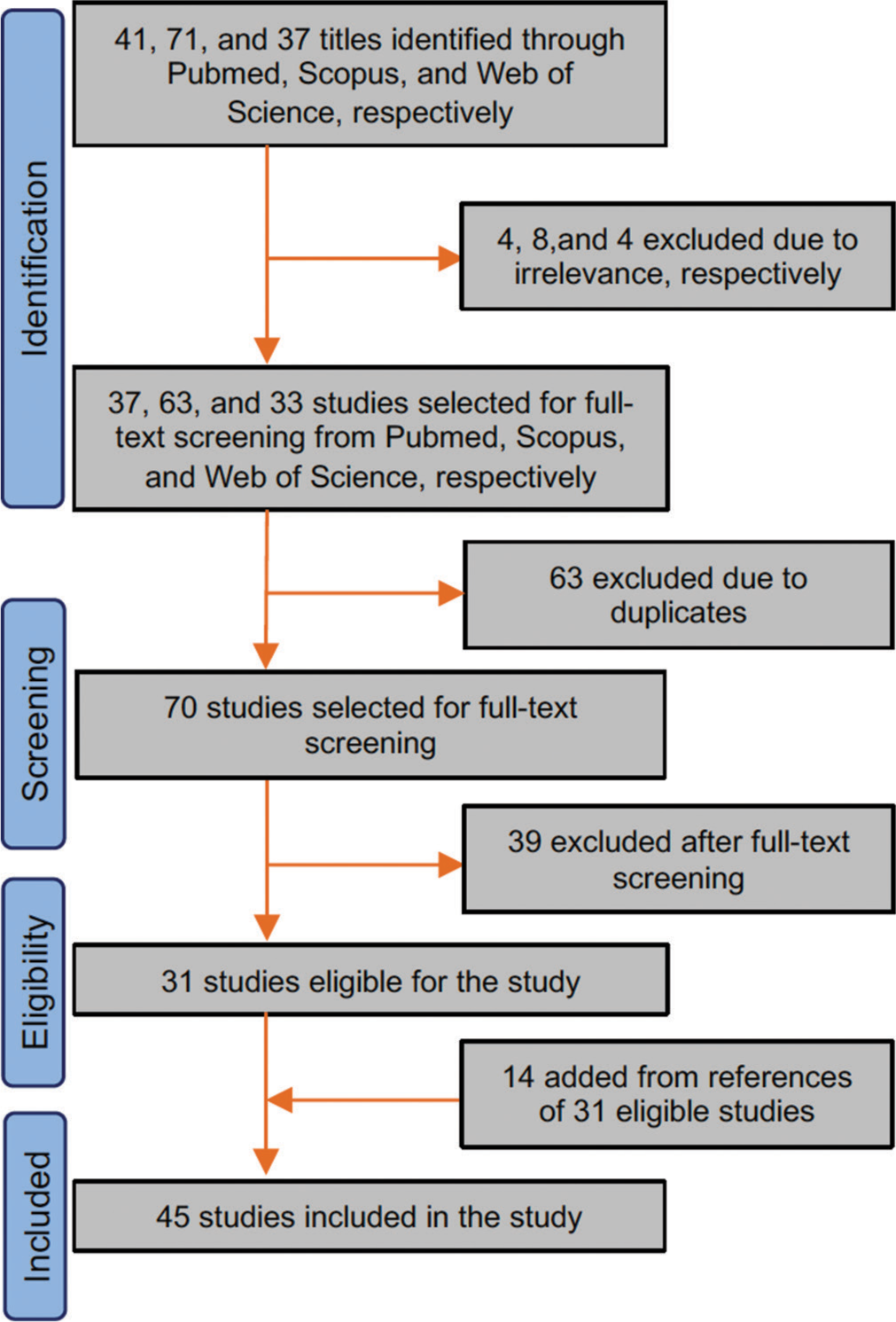
We included 45 studies in the present systematic review after full-text screening [
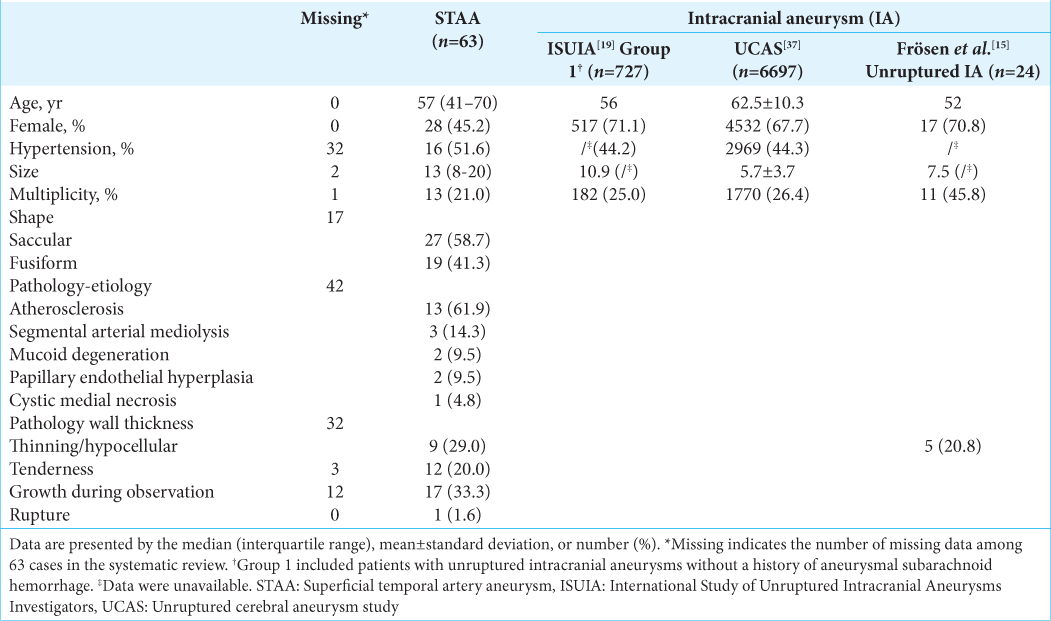
Patients’ and aneurysmal characteristics of STAA and IA are summarized in
Figure 4:
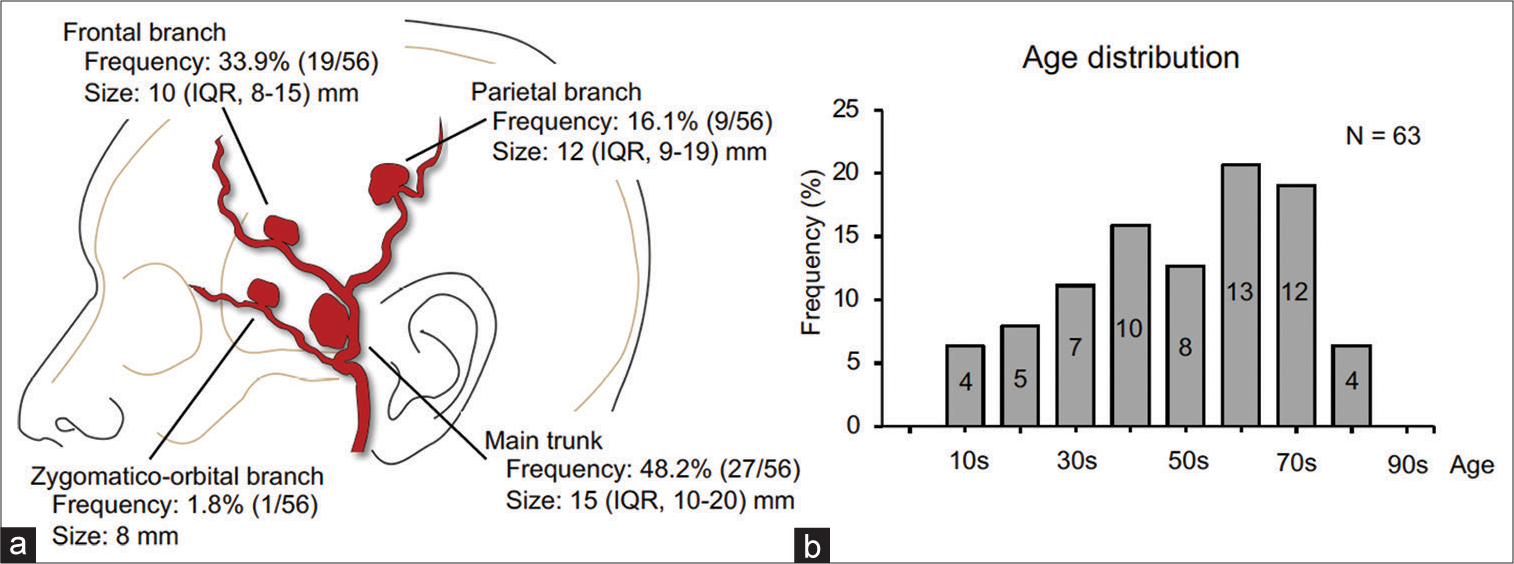
Characteristics of superficial temporal artery aneurysm (STAA) from a systematic review of 63 cases. (a) Incidence and size according to the location of STAA. Data are shown as the median and interquartile range (IQR). Among the 63 cases, data on the location and size are missing in 7 and 2 cases, respectively. (b) Age distribution histogram of patients with STAA. The numbers of patients belonging to each age group divided by every 10 years are shown in the histogram.
Among the characteristics, the similarities of STAA with IA in the International Study of Unruptured IA Investigators and Unruptured Cerebral Aneurysm Study were observed for the patients’ age, the frequency of a history of hypertension, and multiplicity of aneurysms [
DISCUSSION
In this systematic review, nontraumatic true STAA not associated with dissection, infection, or systematic inflammatory diseases was exhaustively included and analyzed. The systematic review of 63 reported cases of STAA showed that most cases were detected as a palpable mass. Intriguingly, aneurysmal rupture was rarely reported, despite the large size of aneurysms (i.e., the median size was 13 mm) and the high frequency of aneurysmal growth during observation.[
The present systematic review showed that several vascular risk factors (i.e., aging and hypertension) were similarly associated with the development of aneurysms both in STA and intracranial arteries [
Segmental arterial mediolysis, which is a rare arteriopathy causing aneurysms in various locations,[
Hemodynamic stress loaded on the arterial wall, which depends on blood flow volume and the morphology of the vessel,[
The prevalence of true saccular aneurysms is diverse between locations throughout the body because of undetermined reasons, which are presumably associated with the pathogenesis. The prevalence of saccular aneurysms is highest in intracranial arteries followed by visceral arteries (e.g., the splenic artery) (3.2% vs. 0.01–0.2%).[
CONCLUSION
To the best of our knowledge, this is the first case to show a rare complication after STA-MCA bypass surgery (i.e., de novo aneurysm formation in the STA). In addition, the present case suggests that the pathogenesis of true STAA is promoted by hemodynamic stress, as with IA. Our systematic review clarified patients’ and aneurysmal characteristics of STAA and treatment outcomes. The analysis of 63 reported cases of STAA suggests that its characteristics are generally in line with that of saccular aneurysms in other locations, such as IA. A male predominance and high frequency of aneurysmal growth during observation in STAA should be regarded as potential differences from IA. This study provides comprehensive data regarding STAA and further insight into the pathogenesis of saccular aneurysms, including STAA and IA.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
SUPPLEMENTARY TABLES
Acknowledgment
We thank Ellen Knapp, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
References
1. Abdelhedy I, Stephen E, Al-Maawali HT, Al-Wahahibi KN. Pulsatile temporal swelling-successful removal of a true superficial temporal artery aneurysm. Indian J Surg. 2020. 82: 725-6
2. Aoki T, Miyata H, Abekura Y, Koseki H, Shimizu K. Rat model of intracranial aneurysm: Variations, usefulness, and limitations of the Hashimoto model. Acta Neurochir Suppl. 2020. 127: 35-41
3. Barrionuevo P, Malas MB, Nejim B, Haddad A, Morrow A, Ponce O. A systematic review and meta-analysis of the management of visceral artery aneurysms. J Vasc Surg. 2019. 70: 1694-9
4. Bor AS, Velthuis BK, Majoie CB, Rinkel GJ. Configuration of intracranial arteries and development of aneurysms: A follow-up study. Neurology. 2008. 70: 700-5
5. Bozkurt G, Ayhan S, Cakici N, Celik O, Ziyal IM. Spontaneous nonpulsatile aneurysm of the superficial temporal artery mimicking a subcutaneous mass lesion. J Craniofac Surg. 2011. 22: 371-2
6. Brinjikji W, Zhu YQ, Lanzino G, Cloft HJ, Murad MH, Wang Z. Risk factors for growth of intracranial aneurysms: A systematic review and meta-analysis. AJNR Am J Neuroradiol. 2016. 37: 615-20
7. Brown R. Aneurysm of the temporal artery. Surgery. 1942. 12: 711-5
8. Brummund D, Chang A, Azimi-Ghomi O, Diaz B, Sendzischew H. Superficial temporal artery true fusiform aneurysm with several lateral feeding vessels. Cureus. 2021. 13: e13973
9. Buckspan RJ, Rees RS. Aneurysm of the superficial temporal artery presenting as a parotid mass. Plast Reconstr Surg. 1986. 78: 515-7
10. Chapman SC, Zak PW, Scaife M, Murdoch G, Eslami MH. Masson tumor (intravascular papillary endothelial hyperplasia) arising in a superficial temporal artery aneurysm. J Vasc Surg cases Innov Tech. 2019. 5: 388-91
11. Coenegrachts K, Hermans R, Poorten VV, Tthijs M, Wilms G. Non-traumatic giant aneurysm of a superficial temporal artery. JBR BTR. 2005. 88: 72-4
12. Dailey RA, Wilson DJ, Putnam D. Superficial temporal-artery aneurysm. Ophthalmic Surg. 1994. 25: 328-9
13. Delen E, Ozkara E, Aydin HE, Ozbek Z. True aneurysm of superficial temporal artery accompanying multiple intracranial aneurysm. Asian J Neurosurg. 2016. 11: 76-7
14. Endo T, Mori K, Maeda M. Multiple arteriosclerotic fusiform aneurysms of the superficial temporal artery--case report. Neurol Med Chir (Tokyo). 2000. 40: 321-3
15. Frösen J, Piippo A, Paetau A, Kangasniemi M, Niemelä M, Hernesniemi J. Remodeling of saccular cerebral artery aneurysm wall is associated with rupture: Histological analysis of 24 unruptured and 42 ruptured cases. Stroke. 2004. 35: 2287-93
16. Frösen J, Tulamo R, Heikura T, Sammalkorpi S, Niemelä M, Hernesniemi J. Lipid accumulation, lipid oxidation, and low plasma levels of acquired antibodies against oxidized lipids associate with degeneration and rupture of the intracranial aneurysm wall. Acta Neuropathol Commun. 2013. 1: 71
17. Gull S, Badawy A, Chaudhuri A The. “pulsatile” sebaceous cyst: Beware of a superficial temporal artery aneurysm. BMJ Case Rep. 2009. 2009: bcr03.2009.1698
18. Ikeda S, Watanabe T. Superficial temporal artery aneurysm associated with pathological changes mimicking cystic medial necrosis. Case report. Neurol Med Chir (Tokyo). 1988. 28: 1223-7
19. International Study of Unruptured Intracranial Aneurysms Investigato. Unruptured intracranial aneurysms--risk of rupture and risks of surgical intervention. N Engl J Med. 1998. 339: 1725-33
20. Joshi D, Klimczak K. Spontaneous rupture of superficial temporal artery aneurysm presenting as hemifacial swelling. BMJ Case Rep. 2014. 2014: bcr2013202308
21. Karam L, El Husseiny M, Abadjian G, Slaba S, Tabet G. Case report of a rare spontaneous superficial temporal artery aneurysm. J Mal Vasc. 2010. 35: 366-8
22. Kato T, Nakamizo M, Yokoshima K, Inai S, Sakanushi A, Ohashi R. Superficial temporal artery aneurysm with abdominal aortic aneurysm: Case report. Jibi Inkoka Tokeibu Geka. 2017. 89: 1111-5
23. Kawabori M, Kuroda S, Nakayama N, Kenmotsu Y, Shimizu H, Tanino M. Spontaneous giant aneurysm of the superficial temporal artery: Case report. Neurol Med Chir (Tokyo). 2009. 49: 198-201
24. Kawai H, Hamasaki T, Imamura J, Tomonori N, Odashiro T, Yamahata H. Three cases of spontaneous superficial temporal artery aneurysm with literature review. Neurol Med Chir (Tokyo). 2014. 54: 854-60
25. Kim E. True aneurysms of the superficial temporal artery: Diagnosis and treatment. Clin Neurol Neurosurg. 2014. 126: 64-8
26. Kitamura S, Horiuchi T. Superficial temporal artery aneurysm with segmental arterial mediolysis: A case report. Br J Neurosurg. 2020. p. Online ahead of print
27. Kotsis T, Christoforou P, Myoteri D, Papacharalampous P. Congenital true aneurysm of the right superficial temporal artery. Med Arch. 2018. 72: 227-9
28. Labropoulos N, Meisner RJ, Gasparis A, Tassiopoulos AK. Management of non-giant cell arteritis disease of the superficial temporal artery. J Vasc Surg. 2011. 53: 200-3
29. Lim JW, Cho KC, Kwak HJ. True aneurysm of the superficial temporal artery. J Korean Neurol Assoc. 2012. 30: 207-9
30. Locatelli D, Messina AL, Ricevuti G, Gajno TM. Superficial temporal artery aneurysms. J Neuroradiol. 1988. 15: 89-93
31. López-Ruiz P, Cuadrado M-L, Aledo-Serrano A, Alonso-Oviés A, Porta-Etessam J, Ganado T. Superficial artery aneurysms underlying nummular headache--2 cases and proposed diagnostic work-up. Headache. 2014. 54: 1217-21
32. Mahajan A, Goel G, Banga V, Das B. An unusual association of hemihypertrophy with extracranial and intracranial aneurysms. Neurol India. 2021. 69: 1481-2
33. Martin WL, Shoemaker WC. Temporal artery aneurysm. Am J Surg. 1955. 89: 700-2
34. Matkovski PD, Filho JO, Candemil PC, Zucco F, Belz WE, Loures JM. Treatment of an atherosclerotic aneurysm of the superficial temporal artery: Case report. J Vasc Bras. 2015. 14: 275-9
35. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009. 6: e1000097
36. Molinaro V, Pagliasso E, Varetto G, Castagno C, Gibello L, Rispoli P. A true aneurysm of the zygomatic orbital artery: First case report in the literature. Case Rep Med. 2012. 2012: 873168
37. Morita A, Kirino T, Hashi K, Aoki N, Fukuhara S, Hashimoto N. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med. 2012. 366: 2474-82
38. Moriyama S, Kunitomo R, Sakaguchi H, Okamoto K, Sasa T, Tanaka M. Intravascular papillary endothelial hyperplasia in an aneurysm of the superficial temporal artery: Report of a case. Surg Today. 2011. 41: 1450-4
39. Mousa AY, Jain AK, Campbell JE, Stone PA, AbuRahma AF. Degenerative (true) superficial temporal artery aneurysm: A case report with review of literature. Vasc Endovasc Surg. 2011. 45: 568-71
40. Nair R, Varsani D, Frecker P. An unusual bump on the head: Spontaneous superficial temporal artery aneurysm. Indian J Surg. 2011. 73: 240-1
41. Nishimura T, Kubota S. A case of congenital AVM in temporoparietal muscle. No Shinkei Geka. 1996. 24: 277-80
42. Nishioka T, Kondo A, Aoyama I, Nin K, Shimotake K, Tashiro H. A case of spontaneous superficial temporal artery aneurysm. No Shinkei Geka. 1988. 16: 1009-12
43. Ohta H, Sakai H, Nakahara I, Sakai N, Nagata I, Ishibashi-Ueda H. Spontaneous superficial temporal artery aneurysm associated with multiple intracranial cerebral aneurysms--does it segmental mediolytic arteriopathy of the intra-and extra-cranial arteries?. Acta Neurochir (Wien). 2003. 145: 805-6
44. Pejkić S, Sladojevic M, Koncar I, Radmili O, Mutavdzic P, Dragaš M. Isolated true aneurysm of the superficial temporal artery: A truly enigmatic lesion. Vasa. 2014. 43: 380-4
45. Piffaretti G, Castelli P. True aneurysms of the superficial temporal artery: Report of three cases. Ann Vasc Surg. 2009. 23: 687.e15-7
46. Porcellini M, Bernardo B, Spinetti F, Carbone F. Outpatient management of superficial temporal artery aneurysms. J Cardiovasc Surg. 2001. 42: 233-6
47. Riaz AA, Ismail M, Sheikh N, Ahmed N, Atkin G, Richman P. Spontaneously arising superficial temporal artery aneurysms: A report of two cases and review of the literature. Ann R Coll Surg Engl. 2004. 86: W38-40
48. Ritz K, Denswil NP, Stam OCG, van Lieshout JJ, Daemen MJ. Cause and mechanisms of intracranial atherosclerosis. Circulation. 2014. 130: 1407-14
49. Sakamoto T, Sugimoto M, Kakigi A, Iwamura H, Kashio A, Suzuki M. A spontaneous true aneurysm of the superficial temporal artery treated by surgical resection. Auris Nasus Larynx. 2011. 38: 119-22
50. Sasaki T, Kakizawa Y, Yoshino M, Fujii Y, Yoroi I, Ichikawa Y. Numerical Analysis of bifurcation angles and branch patterns in intracranial aneurysm formation. Neurosurgery. 2019. 85: E31-9
51. Shimizu K, Imai H, Kawashima A, Okada A, Ono I, Miyamoto S. Induction of CCN1 in growing saccular aneurysms: A potential marker predicting unstable lesions. J Neuropathol Exp Neurol. 2021. 80: 695-704
52. Shimizu K, Kataoka H, Imai H, Miyata T, Okada A, Sakai N. The bifurcation angle is associated with the progression of saccular aneurysms. Sci Rep. 2022. 12: 7409
53. Shimizu K, Kataoka H, Imai H, Yamamoto Y, Yamada T, Miyata H. Hemodynamic force as a potential regulator of inflammation-mediated focal growth of saccular aneurysms in a rat model. J Neuropathol Exp Neurol. 2021. 80: 79-88
54. Shojima M, Oshima M, Takagi K, Torii R, Hayakawa M, Katada K. Magnitude and role of wall shear stress on cerebral aneurysm: computational fluid dynamic study of 20 middle cerebral artery aneurysms. Stroke. 2004. 35: 2500-5
55. Silverberg D, Teodorescu V. True aneurysm of the superficial temporal artery. EJVES Extra. 2005. 9: 126-8
56. Skeik N, Olson SL, Hari G, Pavia ML. Segmental arterial mediolysis (SAM): Systematic review and analysis of 143 cases. Vasc Med. 2019. 24: 549-63
57. Sloane J, Aziz A, Makhdoomi K. A true aneurysm of the superficial temporal artery: Is there an underlying predisposition to such rarities?. Int J Surg Case Rep. 2013. 4: 852-4
58. Uchida N, Sakuma M. Atherosclerotic superficial temporal artery aneurysm: Report of a case. Surg Today. 1999. 29: 575-8
59. Van Uden DJ, Truijers M, Schipper EE, Zeebregts CJ, Reijnen MM. Superficial temporal artery aneurysm: Diagnosis and treatment options. Head Neck. 2013. 35: 608-14
60. Vlak MH, Algra A, Brandenburg R, Rinkel GJ. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: A systematic review and meta-analysis. Lancet Neurol. 2011. 10: 626-36
61. Wei W, Akkersdijk GP. Spontaneous aneurysm of the superficial temporal artery. Lancet. 2011. 378: 168
62. Wise M, Gibson KD. Bilateral superficial temporal artery aneurysms. J Vasc Ultrasound. 2009. 33: 192-4
63. Ysa A, Arruabarrena A, Bustabad MR, Perez E, del Campo A, Garcia-Alonso J. Images in vascular medicine. True aneurysm of the superficial temporal artery. Vasc Med. 2008. 13: 295-6






Dr. Miguel A. Faria
Posted December 10, 2022, 4:58 pm
Interesting case but we reported this back in 1979 in the parent journal of Surgical Neurology International: https://pubmed.ncbi.nlm.nih.gov/524245/