- Department of Neurosurgery, Westmead Hospital, Westmead, New South Wales, Australia
DOI:10.25259/SNI_901_2020
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Heath David French. Tumefactive multiple sclerosis versus high-grade glioma: A diagnostic dilemma. 03-May-2021;12:199
How to cite this URL: Heath David French. Tumefactive multiple sclerosis versus high-grade glioma: A diagnostic dilemma. 03-May-2021;12:199. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=10784
Abstract
Background: Tumefactive demyelinating lesions (TDLs) share similar clinical features and MRI characteristics with high-grade glioma (HGG). This study develops an approach to navigating this diagnostic dilemma, with significant treatment implications as the management of both entities is drastically different.
Methods: A retrospective analysis of 41 TDLs and 91 HGG with respect to demographics, presentation, and classical MRI characteristics was performed. A diagnostic pathway was then developed to help diagnose TDLs based on whole neuraxis MRI and cerebrospinal fluid (CSF) examination.
Results: The diagnosis of TDL is more likely than HGG in younger females who present with subacute or chronic symptoms. MRI characteristics favoring TDL over HGG include smaller size, open rim enhancement, little or no associated edema or mass effect, and the presence of a T2 hypointense rim. MRI of the whole neuraxis for detection of other lesions typical of multiple sclerosis (MS), in combination with a lumbar puncture (LP) showing positive CSF-specific oligoclonal bands (OCB), was positive in 90% of the TDL cohort.
Conclusion: The diagnostic pathway, proposed on the basis of specific clinicoradiological features, should be followed in patients with suspected TDL. If MRI demonstrates other lesions typical of MS and LP demonstrates positive CSF-specific OCBs, then patients should undergo a short course of IV steroids to look for clinical improvement. Patients who continue to deteriorate, do not demonstrate other lesions on MRI or where the LP is negative for CSF-specific OCB, should be considered for biopsy if safe to do so. This pathway will give the patients the best chance at neurological preservation.
Keywords: Biopsy, Diagnostic dilemma, Diagnostic pathway, High-grade glioma, Tumefactive demyelinating lesion, Tumefactive multiple sclerosis
INTRODUCTION
Gross total resection is considered best practice for high-grade glioma (HGG) surgery, however, it may lead to an unnecessary neurological deficit if the histopathology proves to be a tumefactive demyelinating lesion (TDL).[
TDL is classically characterized on MRI by a large solitary lesion with ring enhancement, associated mass effect, and perilesional edema.[
A number of MRI characteristics have been shown in the literature to favor TDL over cerebral neoplasms. These include incomplete rim enhancement, a higher number of lesions, mild or the absence of mass effect and edema, a T2 hypointense rim, and smaller size.[
Using the 2017 McDonald criteria for MS in patients with suspected TDL may help with the differential diagnosis of TDL and HGG. According to the 2017 McDonald criteria, one of the diagnostic imaging hallmarks of MS is that active lesions break the blood brain barrier and enhance when contrast is given, while indolent lesions do not, allowing the identification of disease progression with only one scan. [
Magnetic resonance spectroscopy (MRS) and positron emission tomography (PET) using either fluorodeoxyglucose (FDG) or fluoroethyltyrosine (FET) may be helpful in the diagnosis of TDL, but drawbacks include increased cost and limited availability. In addition, false positives may occur due to the hypercellularity and hypermetabolism of TDL.[
CSF aquaporin 4 antibodies (AQP4-IgG) have been shown to be positive in patients with TDLs in both the CSF and the serum and allow for the alternate demyelinating differential diagnosis of neuromyelitis optica or NMO spectrum disorder (NMO/NMOSD), however, these patients are more likely to have extensive spinal cord lesions.[
This study specifically compares the clinical features and conventional MRI characteristics of TDL with HGG. Knowledge of these key clinicoradiological differences is essential to ensuring the accurate and timely diagnosis of both TDL and HGG. As such, we have developed a diagnostic pathway that allows for identification of patients with TDL, minimizing the risks of unnecessary surgical intervention.
MATERIALS AND METHODS
Aims
The primary aim of this study is to develop a clinicoradiological distinction between TDLs and HGGs. The second is to develop a diagnostic pathway allowing earlier identification of TDLs, in turn, preventing resection associated neurological deficit.
Study design
A retrospective analysis of patients with TDLs was conducted, managed at both the Royal North Shore Hospital (RNSH) and North Shore Private Hospital (NSPH) in Sydney, Australia. The study was approved by the National Health and Medical Research Council. Patients with TDL were drawn from a database of all patients with TDLs managed at the RNSH and NSPH from November 2015 to present. Patients with HGG were randomly drawn from the Sydney Neuro-Oncology Group database of gliomas treated at RNSH from 2014 to present and matched with the TDL group with respect to demographics, presentation, and MRI characteristics. Clinical, laboratory, and radiological data were retrospectively collected.
Demographic characteristics of the cohort pertain to gender and age at the time of first presentation with the cerebral lesion. Symptom type and onset were also recorded. Onset was delineated as acute (<7 days), subacute (>7 days–<3 weeks), and chronic (>3 weeks).
The MRI images were evaluated independently by two neuroradiologists, K.B and C.S.Y.N; with 4 and 5 years of experience in neuroimaging, respectively. Conventional MRI characteristics were standardized in accordance with the previous literature[
Diagnostic criteria for TDL database
Patients over the age of 15 at the time of presentation with at least one lesion on the MRI brain that is larger than 2 cm and with either the presence of mass effect or edema or atypical gadolinium (Gd) enhancement pattern. Patients were excluded if they had a previous diagnosis of MS.
Surgical technique for biopsy of TDL
Tissue biopsies were performed through a focused lesional biopsy technique, using either a stereotactic Cosman-Roberts-Wells frame or a “frameless” navigated biopsy technique for localization.
Statistical analysis
Data were analyzed using Microsoft Excel. Descriptive statistics were mean and percentages for parametric data and median and interquartile range for nonparametric data. Chi-square test and Fisher’s exact test were used to test correlation between categorical variable and Mann–Whitney rank-sum test was used to test correlation between ordinal variables and those without a normal distribution.
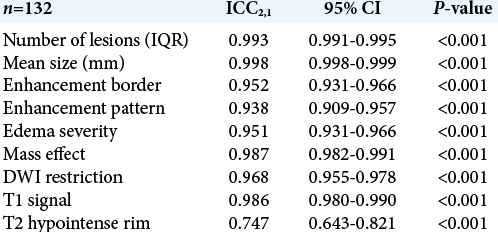
Interclass correlation coefficients for radiological assessments were analyzed on the total cohort (n = 132) using a twoway random effects model for absolute agreement with an alpha set at 95%. An ICC value >0.80 was considered as an excellent agreement. Edema and mass effect were considered as ordinal variables.
A logistical regression model was performed to determine the effects of demographics (age, gender, and symptom duration) and radiological characteristics (T2 hypointense rim and edema) to differentiate between HGG from TDL. Hosmer and Lemeshow goodness-of-fit test was used to test the model.
In all tests, statistical significance was defined as p<0.05. All statistical analyses were completed using SPSS v25.0 (IMB, Armonk, NY).
RESULTS
Clinicoradiological diagnosis of TDLs
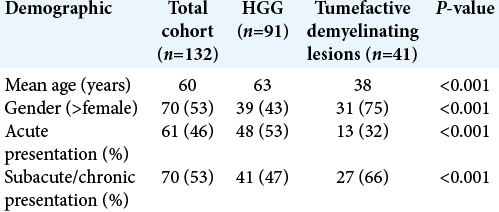
Forty-one TDLs were compared to the 91 HGGs. Univariate analysis of both demographics and clinical features revealed that TDLs were significantly more likely to be present in younger patients and in female patients. Acute presentation was significantly more likely in patients with HGG compared with subacute and chronic in TDLs [
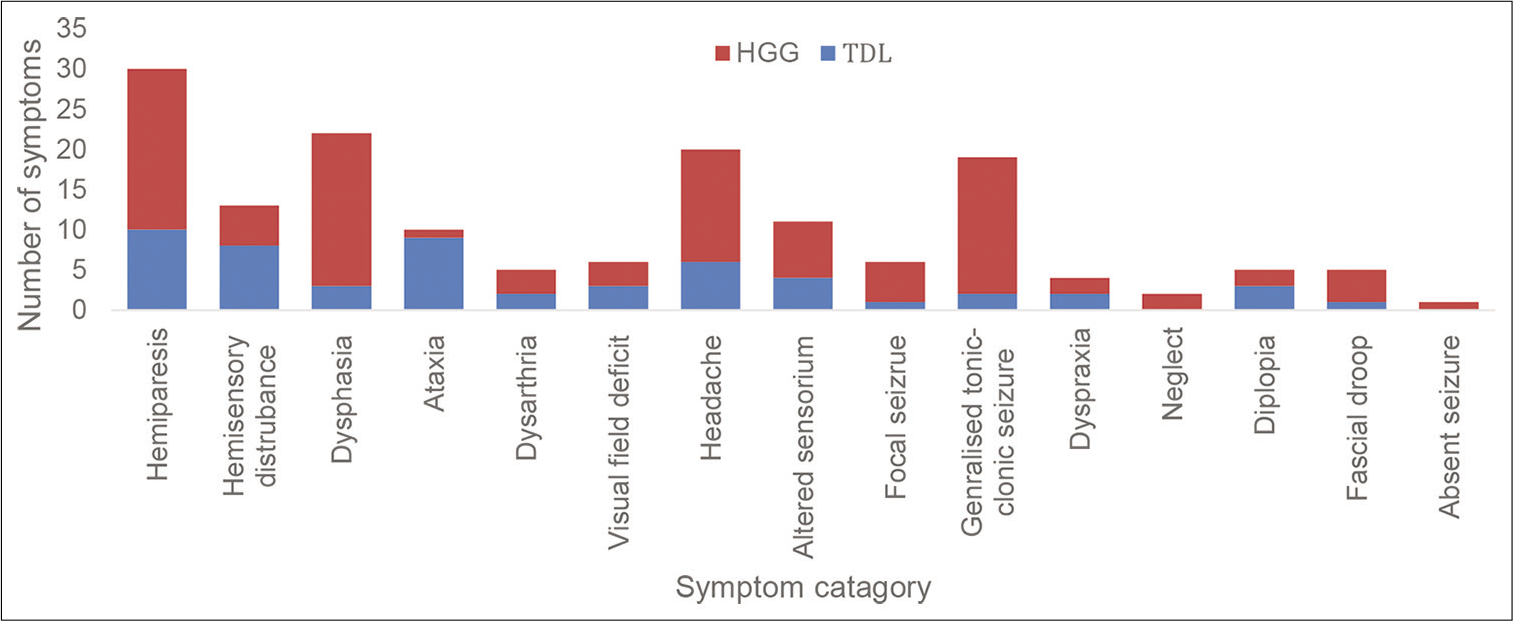
Generalized tonic–clonic (GTC) seizures and dysphasia were more common in patients with HGGs, whereas ataxia, hemi-sensory disturbance, and diplopia were more common in patients with TDLs. Only 1 (3%) patient had a GTC seizure in the TDL group compared with 17 (19%) in the HGG group. Only 3 (7%) patients in the TDL group presented with dysphasia compared with 19 (21%) in the HGG group. Nine (22%) patients presented with ataxia in the TDL group compared with 1 (1%) in the HGG group. Eight (20%) patients with TDLs presented with hemi-sensory disturbance, compared with 5 (5%) in patients with HGG. Three (7%) patients with TDL presented with diplopia compared with 2 (2%) patients with HGG [
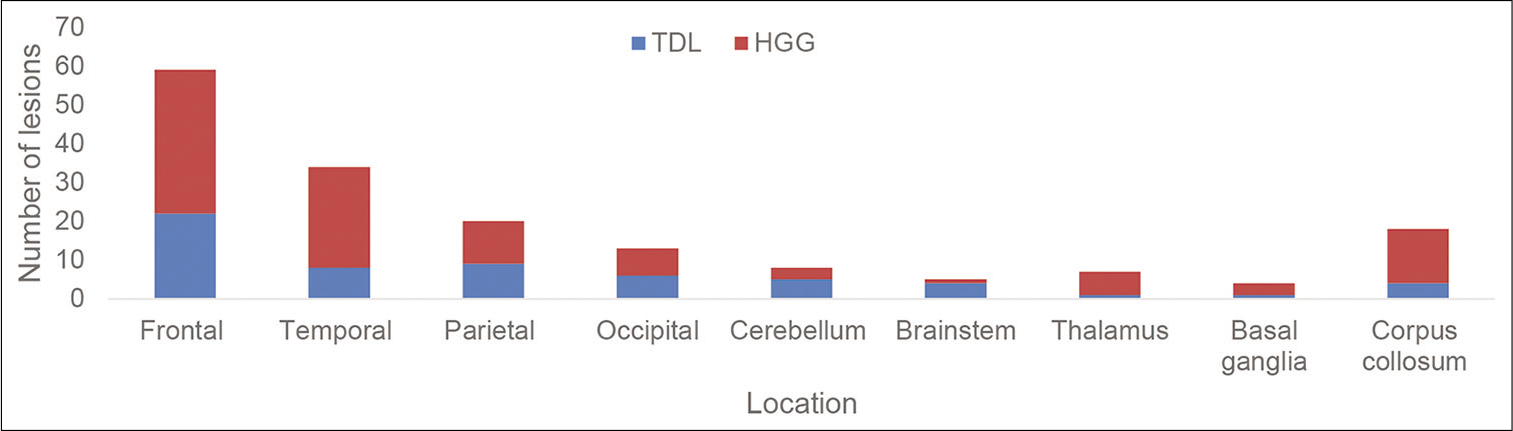
Locations of the lesions were similar between TDLs and HGGs. The most common location was in the frontal lobe followed by the temporal lobe. The brain stem was a more common location in TDLs compared with HGG. Four (10%) TDLs presented in the brainstem compared with 1 (1%) HGG [
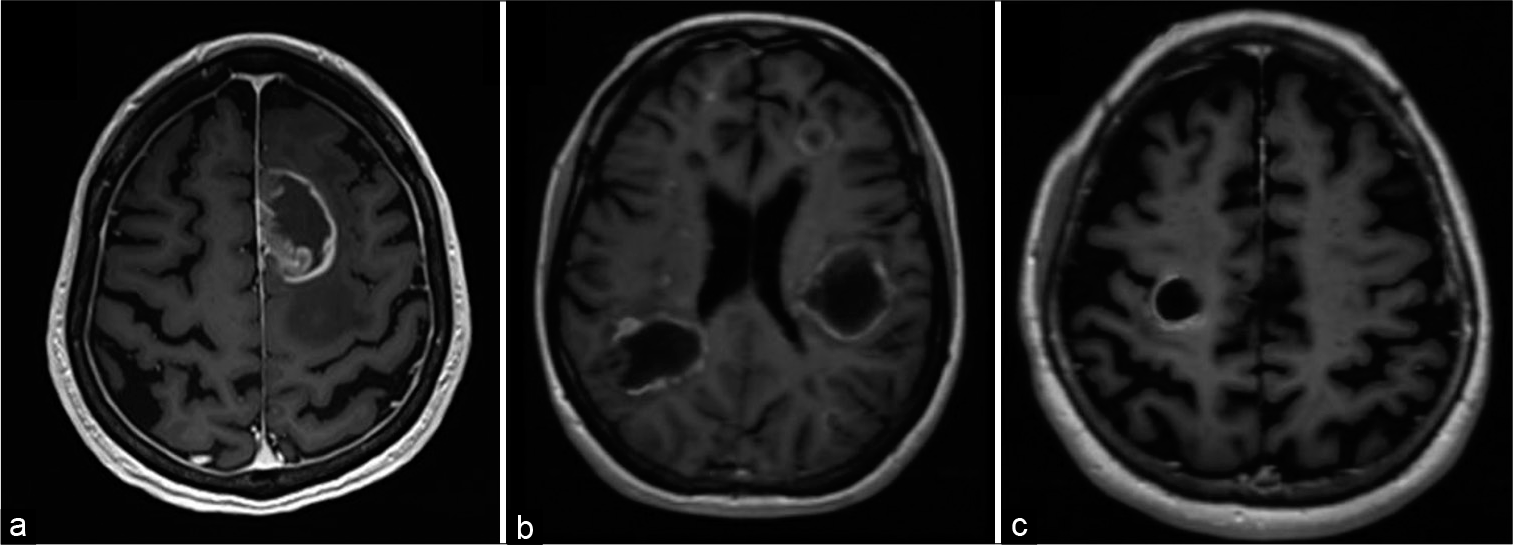
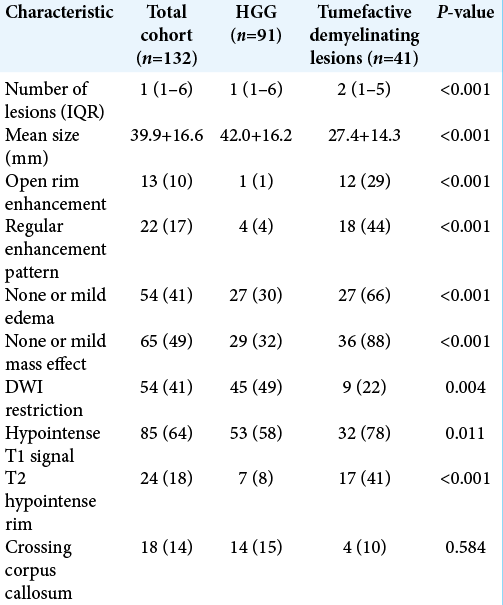
Univariate analysis of conventional MRI characteristics revealed that TDLs, compared with HGGs, were significantly smaller, more likely to have regular and open rim enhancement and demonstrated significantly less associated mass effect and edema [
Figure 3:
(a) Post contrast axial T1 MRI demonstrates irregular closed enhancement of a left frontal high-grade glioma with mild mass effect characterized by sulcal effacement without midline shift. (b) Post contrast axial T1 MRI demonstrates multiple lesions with the right parietal lesion demonstrating open rim enhancement. All lesions demonstrating no mass effect. (c) Post contrast axial T1 MRI demonstrates regular open enhancement of a right frontal TDL with no mass effect or edema.
Figure 4:
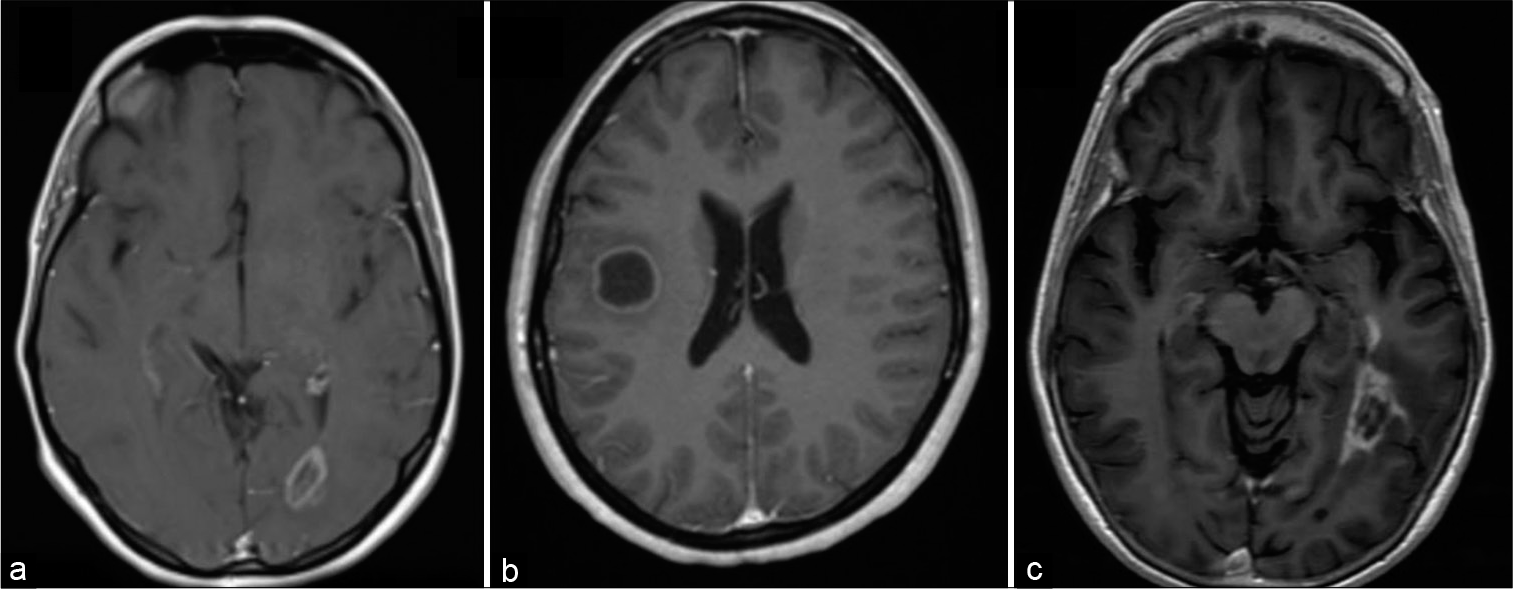
(a) Post contrast axial T1 MRI demonstrates close rim enhancement in a left occipital tumefactive demyelinating lesions (TDLs) with no mass effect or edema. (b) Post contrast axial T1 MRI demonstrates a close regular rim enhancement pattern in a right frontal TDL with minimal mass effect and no edema. (c) Post contrast axial T1 MRI demonstrates irregular closed rim enhancement pattern in a left temporal high-grade glioma with no mass effect.
Figure 5:
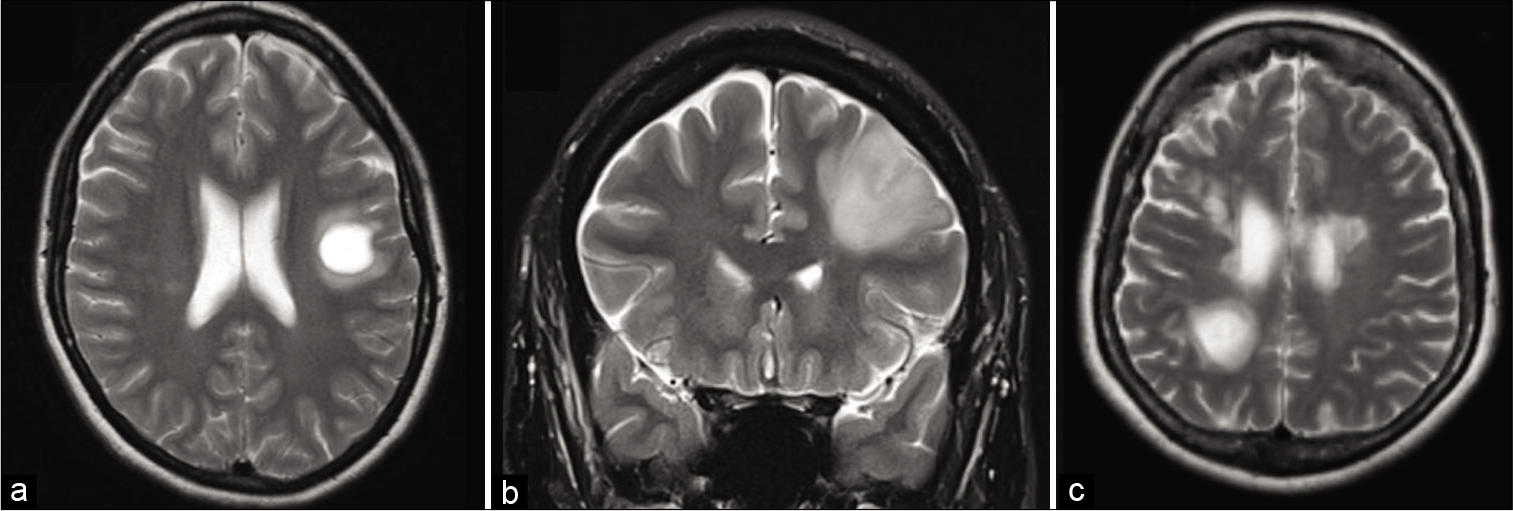
(a) Axial T2 FLAIR of a left frontal tumefactive demyelinating lesion (TDL) showing minimal perilesional edema. There are lesions characteristic of MS in the right periventricular region. (b) Axial T2 FLAIR in a high-grade glioma in the right temporal lobe with mixed T2 signal reflecting a combination of lesional necrosis, hemorrhage, and neovascularity. There is moderate perilesional edema. (c) Axial T2 MRI of a right frontal TDL demonstrating no neovascularity, minimal perilesional edema, and no mass effect.
TDLs were also more likely to have a T2 hypointense rim and a hypointense T1 signal [
Figure 6:
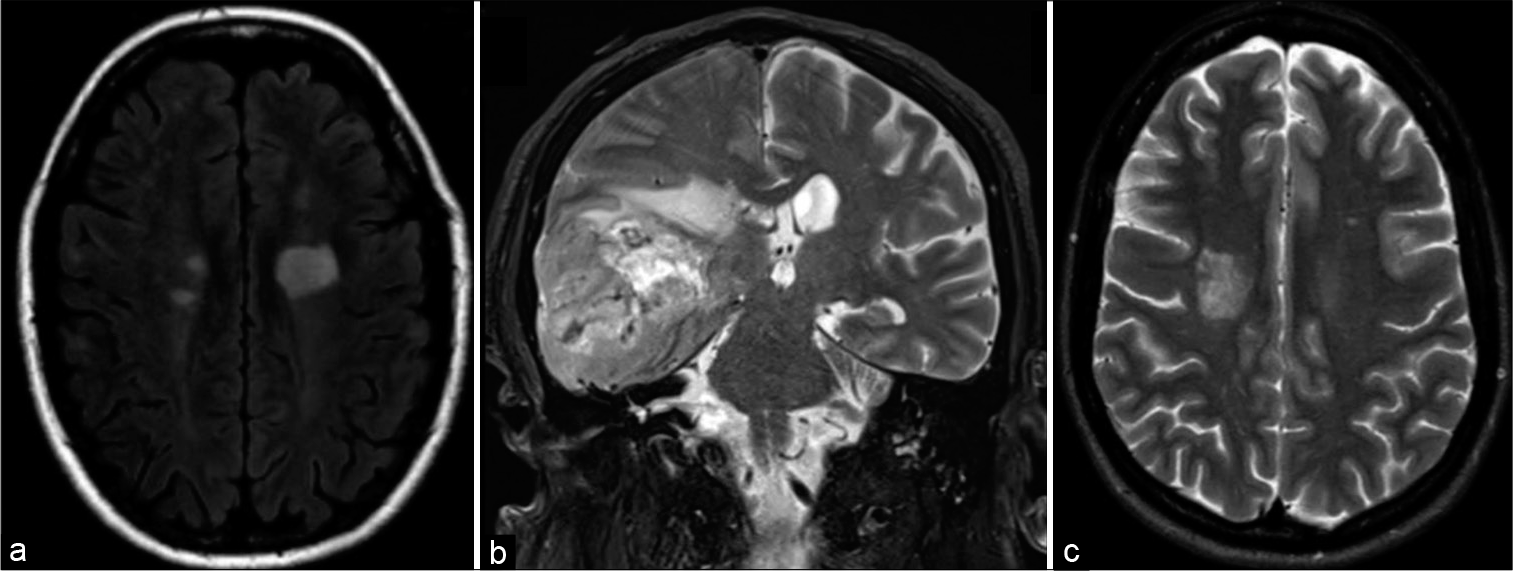
(a) T2 axial MRI demonstrates a T2 hypointense rim in a left frontal tumefactive demyelinating lesion (TDL) with mild edema and no mass effect. (b) T2 corona MRI demonstrates the absence of a T2 hypointense rim in the left frontal high-grade glioma. (c) T2 axial MRI demonstrates a 0–2 hypointense rim in a right parietal TDL with mild edema and mass effect.
Using the aforementioned MRI characteristics described, high inter-rater reliability between radiologists was demonstrated. All demonstrated high interclass correlation coefficients [
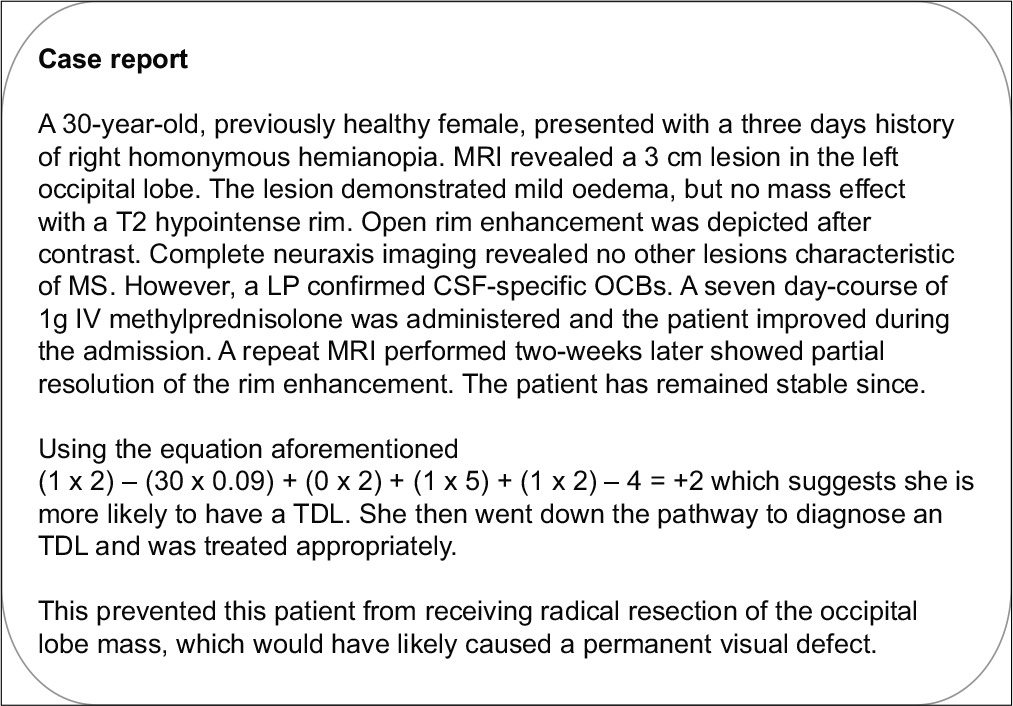
A logistical regression model was created using the clinicoradiological characteristics that would best differentiate TDLs from HGGs. The logistic regression model was statistically significant c2 (5) = 107.819, P < 0.001. The model correctly classified 88.5% of cases and correctly diagnosed TDLs 82.5% of the time. Using the regression coefficients from the logistical regression analysis, an equation that predicts patients who are more likely to have TDLs compared with HGGs based on the clinicoradiological characteristics in the model was created. It was then simplified to make it easy to work with and apply in the outpatients setting. Variables are binary except age, that is, female 1 and male 0, presentation >7 weeks 1 and presentation <7 weeks 0, none to mild edema 1 and moderate to severe edema 0, and presence of T2 hypointense rim 1 and if absent 0. If x in the equation is positive, then the patient is more likely to have a TDL and if negative the patient is more likely to have an HGG.
Equation to predict TDL over HGG
(gender x 2) – (age x 0.05) + (presentation > 7 weeks x 2) + (none or mild edema x 5) + (T2 hypointense rim x 2) – 4 = x
Diagnostic pathway in patients with suspected TDLs
Thirty-one (76%) patients with TDLs had other lesions characteristic of MS on the MRI brain (periventricular, juxtacortical, infratentorial, and spinal cord) [
Figure 7:
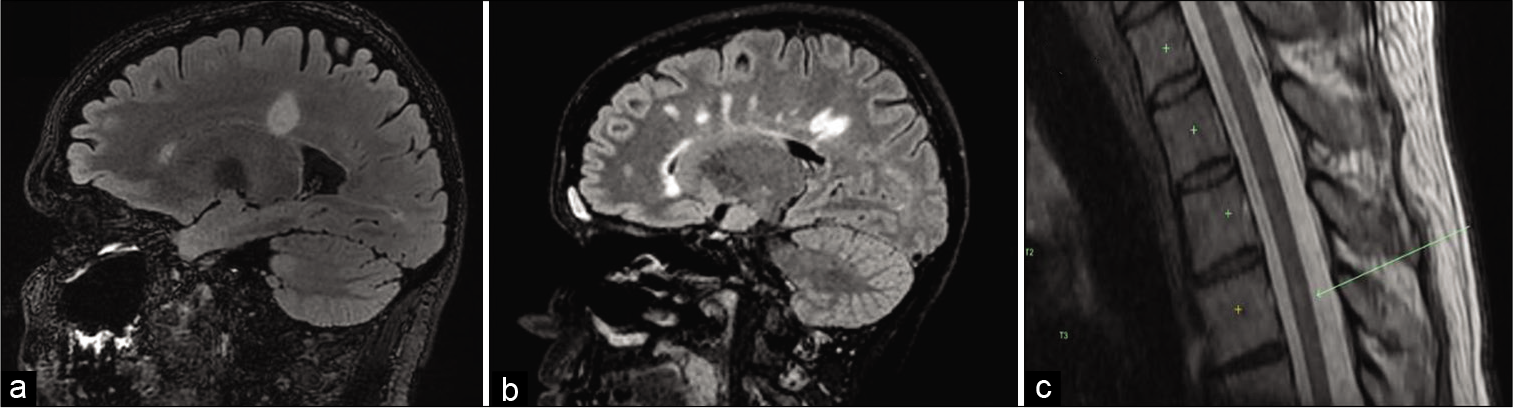
(a) FLAIR sagittal MRI demonstrates lesions typical of tumefactive demyelinating lesions (TDLs) periventricular lesions. (b) T2 FLAIR sagittal MRI demonstrates lesions typical of TDL and MS (Dowson’s fingers). (c) T2 spine MRI demonstrates subtle T2 hyperintense lesion in the thoracic spinal cord typical of a patient with an TDL.
Thirty-seven (90%) patients out of the cohort satisfied the McDonald criteria for the diagnosis of MS. For every patient in the cohort, it was their first presentation and therefore no patient satisfied the criteria by DIT clinically. In five patients that did not demonstrate lesions typical of MS, the presence of CSF-specific OCB satisfied the McDonald criteria. In three patients, the CSF was not examined. Only one patient who did not have lesions typical of MS on their whole neuraxis was negative for CSF-specific OCBs.
Chi-squared test between three variables (CSF-specific OCB, other lesions typical of MS on brain MRI, and spinal lesions on MRI) showed no correlation between the variables and that each variable was sufficiently independent.
CSF protein was elevated above 0.45 g/L in two patients to 0.62 g/L and in all patients, glucose was within normal limits (2.5–5.6 mmol/L). The occasional monocyte was seen in all patients. In 13 patients, the CSF was examined for aquaporin four antibodies and only 2 (15%) were positive. In the four patients, where the CSF was tested for myelin oligodendrocyte glycoprotein antibodies, all were negative.
Three patients underwent an FDG PET study, with only one case depicting mildly increased FDG avidity. FET PET was performed in one patient and the TDL did not demonstrate avidity.
Biopsy
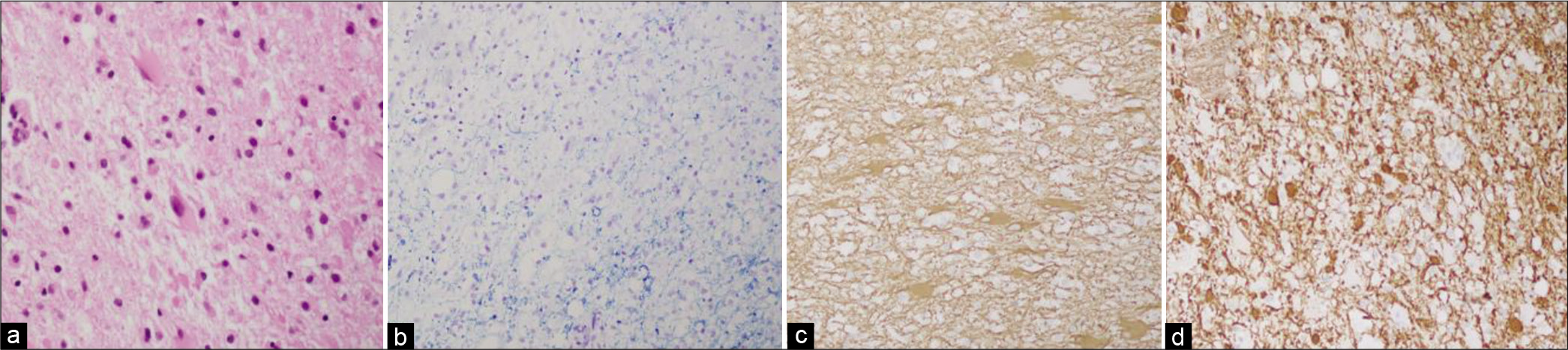
Five patients underwent biopsy. The hematoxylin and eosin stains revealed foamy macrophage infiltrate and perivascular lymphocytic inflammation of the white matter. Luxol fast blue, myelin basic protein, and neurofilament protein stain revealed demyelination. Glial fibrillary acidic protein shows gemistocytic cells (reactive gliosis). Tumor markers such as isocitrate dehydrogenase 1 were not seen. No malignant cells or organisms were identified in any of the biopsies [
Figure 8:
(a) H&E stain that demonstrates foamy macrophages with reactive gliosis. (b) Luxol fast blue stain that demonstrates the absent of myelin (blue). (c) Glial fibrillary acidic protein stain that demonstrates reactive gliosis (gemistocytes). (d) Neurofilament protein stain that demonstrates demyelination.
All biopsied patients in our cohort satisfied the McDonald criteria. Out of these five patients, four underwent LP and only two patients were positive for CSF-specific OCB. Reasons for biopsy in these cases were negative CSF-specific OCB, lack of awareness of TDL by the treating clinician, profound neurological deficit on presentation, and to rule out infection in one patient.
Management and outcome
Thirty-three (79%) patients were treated with intravenous methylprednisolone for 5–7 days, and one patient was treated with a course of oral steroids. Of the patients who received methylprednisolone, most (82%) showed a partial or significant improvement in their symptoms. Five (12%) patients showed complete resolution of their symptoms. One patient developed significant improvement despite no specific treatment.
DISCUSSION
Clinicoradiological diagnosis of TDLs
TDL shows a predilection for females and tends to occur in middle-aged individuals.[
Symptomatology in TDLs is distinctly different to classical MS and more commonly seen in patients with HGG.[
The most common imaging modality for both TDL and HGG is a conventional MRI.[
TDL and HGG both demonstrate a predilection for the frontal and temporal lobes, which is consistent with our findings in this study. Consequently, the locations do not add value when attempting to differentiate between TDL and HGG.[
Diagnostic work-up of patients with suspected TDL
About 90% of patients met the 2017 McDonald criteria in our cohort. Out of these, 76% of patients had other lesions classical for MS on the MRI brain, including white matter lesions around the ventricles and juxtacortical, in the brainstem, and/or in the cerebellum.[
A LP, to allow for CSF analysis, is a simple outpatient investigation that should accompany full imaging studies in patients with suspected TDL, if safe to do so. About 74% of patients with TDL were positive for CSF-specific OCBs. In the literature, CSF-specific OCB has been demonstrated in 30–80% of patients with TDL.[
CSF-AQP4 IgG has been shown to help diagnose NMO/ NMOSD in patients with TDL.[
FDG-PET and FET-PET were done on a limited number of patients in this cohort and did not help differentiate TDL from HGG. This is in part due to their cost, availability, and novelty in our centers for the work-up of TDL. As aforementioned, TDL has been shown to demonstrate avidity on FDG-PET and FET-PET secondary to hypercellularity and inflammation, thus resulting in false positives.[
Biopsy should be reserved for diagnostically challenging cases in which, despite the aforementioned investigations, the diagnosis of demyelinating lesion cannot be made. Should a pathological diagnosis be required, biopsy should ideally precede resection to give the patient the best chance at neurological preservation, especially when resection may result in permanent neurological deficits.[
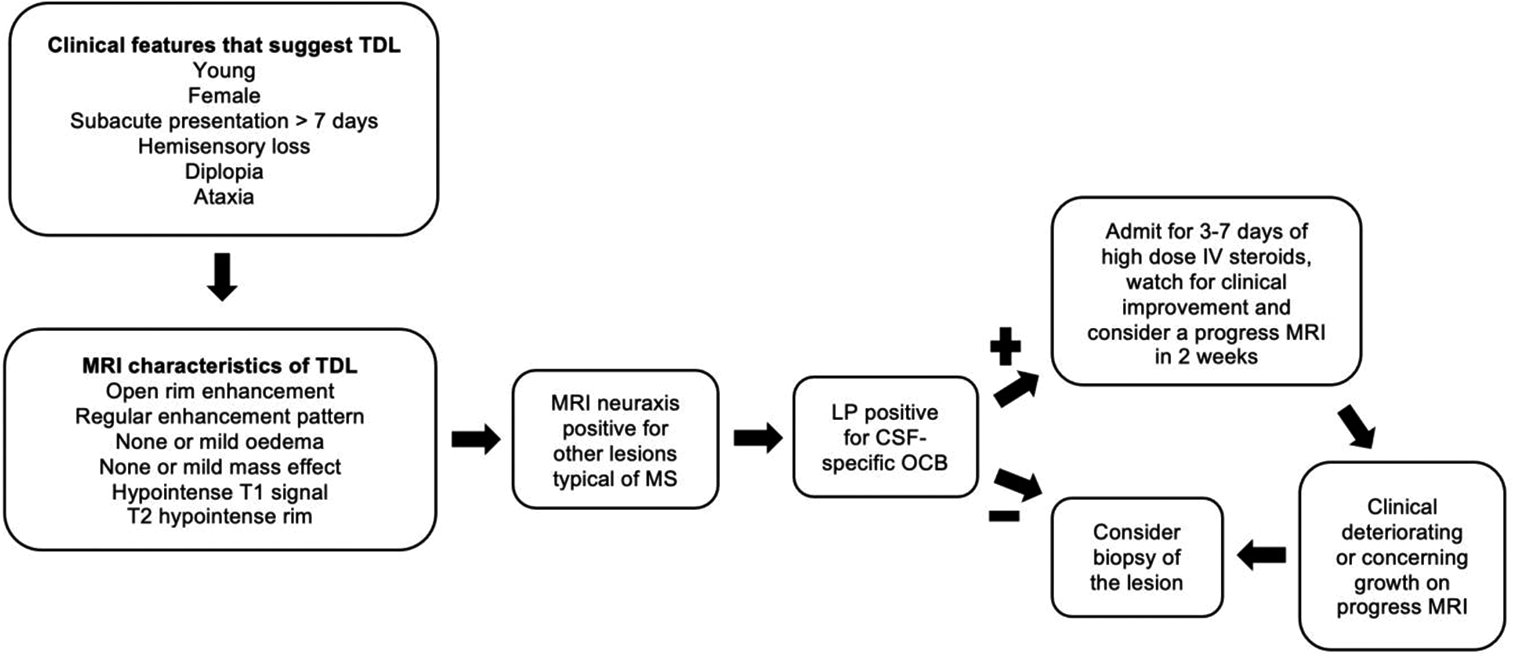
Preliminary diagnostic pathway for patients with a suspected TDL
The findings of our study support further research into a pathway that will assist clinicians in the diagnosis of TDL [
Limitations
This study is limited by the retrospective design and data collection being dependent on medical records. Limitations of medical record review include chart ambiguity, omissions, and data entry error. An attempt to minimize these limitations was sought through a simple, exact, nonambiguous data collection tool, and an attempt to define ambiguous terms. This study did not analyze other neoplasms or infections that TDL could mimic and therefore cannot be generalized to this differential diagnosis. In an attempt to minimize the effect of selection bias secondary to comparing diseases with different incidences, a random selection of 91 patients from the SNOG database treated from 2014 to present was compared with 41 TDL accumulated in the same timeframe.
CONCLUSION
TDLs are an unusual manifestation of MS that shares a number of clinicoradiological features with HGGs. This has made TDL diagnosis historically difficult. This study demonstrates a clinicoradiological distinction between TDLs and HGGs. Specifically, a TDL is a more likely diagnosis in younger females presenting with subacute or chronic symptoms, with a lesion on MRI that demonstrates no to mild edema and a T2 hypointense rim. As demonstrated in the proposed diagnostic pathway, these features should prompt clinicians to image the whole neuraxis and perform a LP. If the MRI demonstrates other lesions typical of MS and the LP demonstrates oligoclonal bands, then the patient should undergo a short course of IV steroids. In patients who do not meet criteria for a trial of steroids, or in those patients who deteriorate despite steroid therapy, a biopsy should be considered. Adoption of this pathway is likely to minimize unnecessary resection, with its associated neurological sequelae.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Abdoli M, Freedman MS. Neuro-oncology dilemma: Tumour or tumefactive demyelinating lesion. Mult Scler Relat Disord. 2015. 4: 555-66
2. Altintas A, Petek B, Isik N, Terzi M, Bolukbasi F, Tavsanli M. Clinical and radiological characteristics of tumefactive demyelinating lesions: Follow-up study. Mult Scler. 2012. 18: 1448-53
3. Cotton F, Weiner HL, Jolesz FA, Guttmann CR. MRI contrast uptake in new lesions in relapsing-remitting MS followed at weekly intervals. Neurology. 2003. 60: 640-6
4. Filss CP, Cicone F, Shah NJ, Galldiks N, Langen KJ. Amino acid PET and MR perfusion imaging in brain tumours. Clin Transl Imaging. 2017. 5: 209-23
5. Jeong IH, Kim SH, Hyun JW, Joung A, Cho HJ, Kim HJ. Tumefactive demyelinating lesions as a first clinical event: Clinical, imaging, and follow-up observations. J Neurol Sci. 2015. 358: 118-24
6. Kaeser MA, Scali F, Lanzisera FP, Bub GA, Kettner NW. Tumefactive multiple sclerosis: An uncommon diagnostic challenge. J Chiropr Med. 2011. 10: 29-35
7. Kebir S, Gaertner FC, Mueller M, Nelles M, Simon M, Schäfer N. 18F-fluoroethyl-L-tyrosine positron emission tomography for the differential diagnosis of tumefactive multiple sclerosis versus glioma: A case report. Oncol Lett. 2016. 11: 2195-8
8. Kim DS, Na DG, Kim KH, Kim J, Kim E, Yun BL. Distinguishing tumefactive demyelinating lesions from glioma or central nervous system lymphoma: Added value of unenhanced CT compared with conventional contrast-enhanced MR imaging. Radiology. 2009. 251: 467-75
9. Lin X, Yu WY, Liauw L, Chander RJ, Soon WE, Lee HY. Clinicoradiologic features distinguish tumefactive multiple sclerosis from CNS neoplasms. Neurol Clin Pract. 2017. 7: 53-64
10. Liu JG, Qiao WY, Qi XK, Zhao HL, Zheng KH, Qian HR. Comparison of the features of MRI of tumefactive demyelinating lesions and glioma. J Neurol Sci. 2015. 357: e15-6
11. Lucchinetti CF, Gavrilova RH, Metz I, Parisi JE, Scheithauer BW, Weigand S. Clinical and radiographic spectrum of pathologically confirmed tumefactive multiple sclerosis. Brain. 2008. 131: 1759-75
12. Maarouf M, Kuchta J, Miletic H, Ebel H, Hesselmann V, Hilker R. Acute demyelination: Diagnostic difficulties and the need for brain biopsy. Acta Neurochir (Wien). 2003. 145: 961-9
13. Mabray MC, Cohen BA, Villanueva-Meyer JE, Valles FE, Barajas RF, Rubenstein JL. Performance of apparent diffusion coefficient values and conventional MRI features in differentiating tumefactive demyelinating lesions from primary brain neoplasms. AJR Am J Roentgenol. 2015. 205: 1075-85
14. Mauri-Fábrega L, Díaz-Sánchez M, Casado-Chocán JL, UclésSánchez AJ. Pseudotumoral forms of multiple sclerosis: Report of 14 cases and review of the literature. Eur Neurol. 2014. 72: 72-8
15. McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD. Recommended diagnostic criteria for multiple sclerosis: Guidelines from the international panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001. 50: 121-7
16. Ostrom QT, Bauchet L, Davis FG, Deltour I, Fisher JL, Langer CE. The epidemiology of glioma in adults: A “state of the science” review. Neuro Oncol. 2014. 16: 896-913
17. Pirko I, Gamez J, Johnson AJ, Macura SI, Rodriguez M. Dynamics of MRI lesion development in an animal model of viral-induced acute progressive CNS demyelination. Neuroimage. 2004. 21: 576-82
18. Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol. 2005. 58: 840-6
19. Popescu BF, Guo Y, Jentoft ME, Parisi JE, Lennon VA, Pittock SJ. Diagnostic utility of aquaporin-4 in the analysis of active demyelinating lesions. Neurology. 2015. 84: 148-58
20. Rutka J, Nunez O, Seol H. The role of surgery in the management of intracranial gliomas: Current concepts. Indian J Cancer. 2009. 46: 120-6
21. Sajja BR, Wolinsky JS, Narayana PA. Proton magnetic resonance spectroscopy in multiple sclerosis. Neuroimaging Clin N Am. 2009. 19: 45-58
22. Schwartz KM, Erickson BJ, Lucchinetti C. Pattern of T2 hypointensity associated with ring-enhancing brain lesions can help to differentiate pathology. Neuroradiology. 2006. 48: 143-9
23. Suh CH, Kim HS, Jung SC, Choi CG, Kim SJ. MRI findings in tumefactive demyelinating lesions: A systematic review and meta-analysis. AJNR Am J Neuroradiol. 2018. 39: 1643-9
24. Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018. 17: 162-73
25. Toh CH, Wei KC, Ng SH, Wan YL, Castillo M, Lin CP. Differentiation of tumefactive demyelinating lesions from high-grade gliomas with the use of diffusion tensor imaging. AJNR Am J Neuroradiol. 2012. 33: 846-51
26. Xia L, Lin S, Wang Z, Li S, Xu L, Wu J. Tumefactive demyelinating lesions: Nine cases and a review of the literature. Neurosurg Rev. 2009. 32: 171-9