- Department of Neurosurgery, Fukuoka Children’s Hospital, Saga, Japan.
- Department of Neurosurgery, Harasanshin Hospital, Saga, Japan.
- Department of Neurosurgery, Kyushu University, Saga, Japan.
- Department of Dermatology, Fukuoka Children’s Hospital, Saga, Japan.
- Department of Psychiatry, Shourai Hospital, Saga, Japan.
Correspondence Address:
Ai Kurogi
Department of Neurosurgery, Kyushu University, Saga, Japan.
DOI:10.25259/SNI_626_2020
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ai Kurogi1, Nobuya Murakami1, Takato Morioka2, Nobutaka Mukae3, Takafumi Shimogawa3, Kyoko Kudo4, Satoshi O. Suzuki5, Masahiro Mizoguchi3. Two cases of retained medullary cord running parallel to a terminal lipoma. 24-Mar-2021;12:112
How to cite this URL: Ai Kurogi1, Nobuya Murakami1, Takato Morioka2, Nobutaka Mukae3, Takafumi Shimogawa3, Kyoko Kudo4, Satoshi O. Suzuki5, Masahiro Mizoguchi3. Two cases of retained medullary cord running parallel to a terminal lipoma. 24-Mar-2021;12:112. Available from: https://surgicalneurologyint.com/surgicalint-articles/10667/
Abstract
Background: Retained medullary cord (RMC) is a newly defined entity believed to originate from the late arrest of secondary neurulation. Some RMCs contain varying amounts of lipomatous tissues, which need to be differentiated from spinal lipomas, such as filar and caudal lipomas (terminal lipomas).
Case Description: We surgically treated two patients with a nonfunctional cord-like structure (C-LS) that was continuous from the cord and extended to the dural cul-de-sac, and ran parallel to the terminal lipoma. In both cases, untethering surgery was performed by resecting the C-LS with lipoma as a column, under intraoperative neurophysiological monitoring. Histopathological examination confirmed that the central canal-like ependyma-lined lumen with surrounding neuroglial and fibrocollagenous tissues, which is the central histopathological feature of an RMC, was located on the unilateral side of the resected column, while the fibroadipose tissues of the lipoma were located on the contralateral side.
Conclusion: Our findings support the idea proposed by Pang et al. that entities such as RMC and terminal lipomas are members of a continuum of regression failure occurring during late secondary neurulation, and the coexistence of RMC and terminal lipoma is not a surprising finding. Therefore, it may be difficult in clinical practice to make a distinct diagnosis between these two entities.
Keywords: Retained medullary cord, Secondary neurulation, Terminal lipoma
INTRODUCTION
Retained medullary cord (RMC) is a newly defined entity believed to originate from an almost complete arrest of apoptosis during the last or degenerative phase of secondary neurulation.[
Although RMCs are not considered very rare,[
CASE REPORT
Patient 1
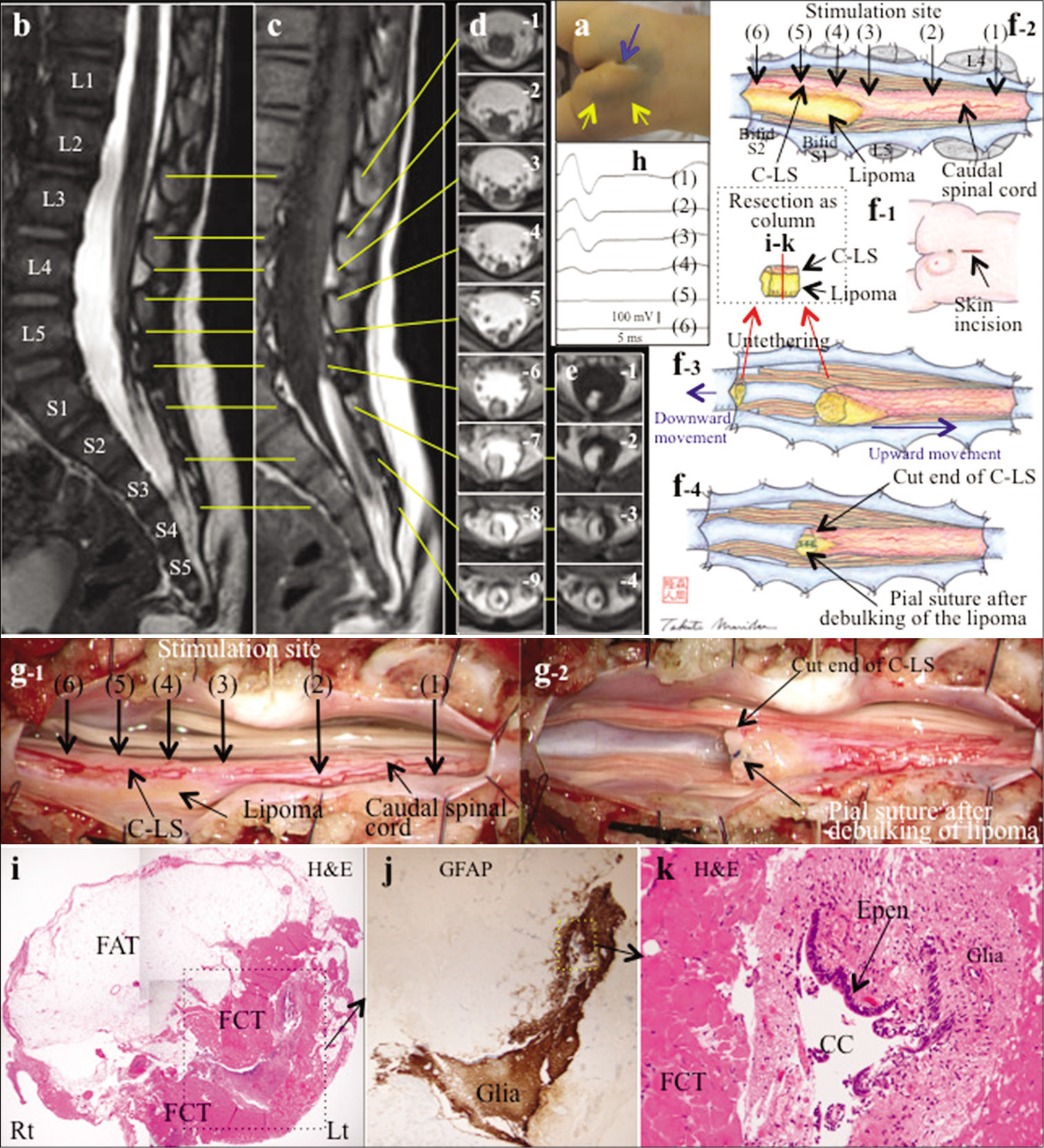
Patient 1 was a boy without neurological deficits. At birth, he had a small tail-like appendage overlying a subcutaneous mass on the right side of the gluteal cleft, which was resected by a plastic surgeon a few days after his birth. His parents noticed that the subcutaneous mass gradually increased in size, and he was referred to us at the age of 1 year and 6 months. His gluteal cleft deviated to the left due to the subcutaneous mass [
Figure 1:
(a) Photograph showing a subcutaneous mass (yellow arrows) on the right side of the gluteal cleft which deviates to the left. Another groove is noted at the rostral side of the gluteal cleft (blue arrow). (b-d) Sagittal views (slice thickness of 1.25 mm) of three-dimensional heavily T2-weighted image (3D-hT2WI) (b) and three-dimensional T1-weighted spoiled gradient-recalled echo image (3D-T1WI) (c) and axial views (slice thickness of 5.2 mm) of T2-weighted image (T2WI) depict that the caudal spinal cord remains thick with minimal tapering (b, c, d-1-5) and, with minimal increase in diameter, extends as a cord-like structure (C-LS) to the dural cul-de-sac at the L3-4 vertebral level (b, c, and d-6-9). (e) Axial views (slice thickness of 5.2 mm) of 3D-T1WI demonstrate that lipoma is associated with the right half of the C-LS (e1-3). The extradural C-LS is located in the center of the epidural fat (e-4). (f) Schematic drawing and (g) microscopic view of the operative findings and (h) intraoperative neurophysiological monitoring (IONM). A lineal skin incision was made on the mid-lumbosacral region (f-1). Laminoplastic laminotomy of L4-bifid S2 revealed that the caudal spinal cord and C-LS extended to the dural cul-de-sac without an intervening terminal filum (f-2, g-1). The lipoma was observed at the right side of the C-LS below L5-S1 level. The border between the true cord and C-LS was determined with IONM, by tracing the evoked compound muscle action potentials (CMAPs) of the external anal sphincter with stimulation of 1 mA (h), beginning from the functional cord (h-(1)(2)(3)) and proceeding to the to the nonfunctional CL-S (h-(4)(5) (6)). Stimulation sites are indicated on (f-2) and (g-1). The C-LS with lipoma was severed at this border and most caudal side of the operative field and resected as a column (f-3). The lipoma was minimally debulked and the pial surface was reconstructed with sutures (f-4, g-2). (i-k) Photomicrograph of cross-sections of the C-LS including lipoma with hematoxylin and eosin staining (HE) (i,k) and immunostaining for glial fibrillary acidic protein (GFAP) (j). The location of section is indicated as red line in (f-3) and the orientation is almost matched with that of (e-2). A higher magnification view of the area is indicated by the dotted square in (i) and (j). The C-LS, which consists of fibrocollagenous tissue (FCT) embedding a central canal (CC)-like structure lined by ependymal cells (Epen) and surrounded by GFAP immunopositive neuroglial tissues, is located on the left side of the resected column. The lipoma, which consists of a mature fibroadipose tissue (FAT), is located on the right side of the C-LS. Original magnification: (i) ×4, (j) ×40, and (k) ×200.
Untethering surgery was performed at the age of 1 year and 8 months. Laminoplastic laminotomy of L4-bifid S2 revealed that the caudal spinal cord and C-LS extended to the dural cul-de-sac without an intervening terminal filum. The lipoma was observed at the right side of the C-LS below the L5-S1 level [
The postoperative course was uneventful. Histopathological examination revealed that the C-LS, which consisted of fibrocollagenous tissue embedding a central canal-like structure lined by ependymal cells and surrounded by glial fibrillary acidic protein (GFAP)-immunopositive neuroglial tissues, was located on the left side of the resected column [
Patient 2
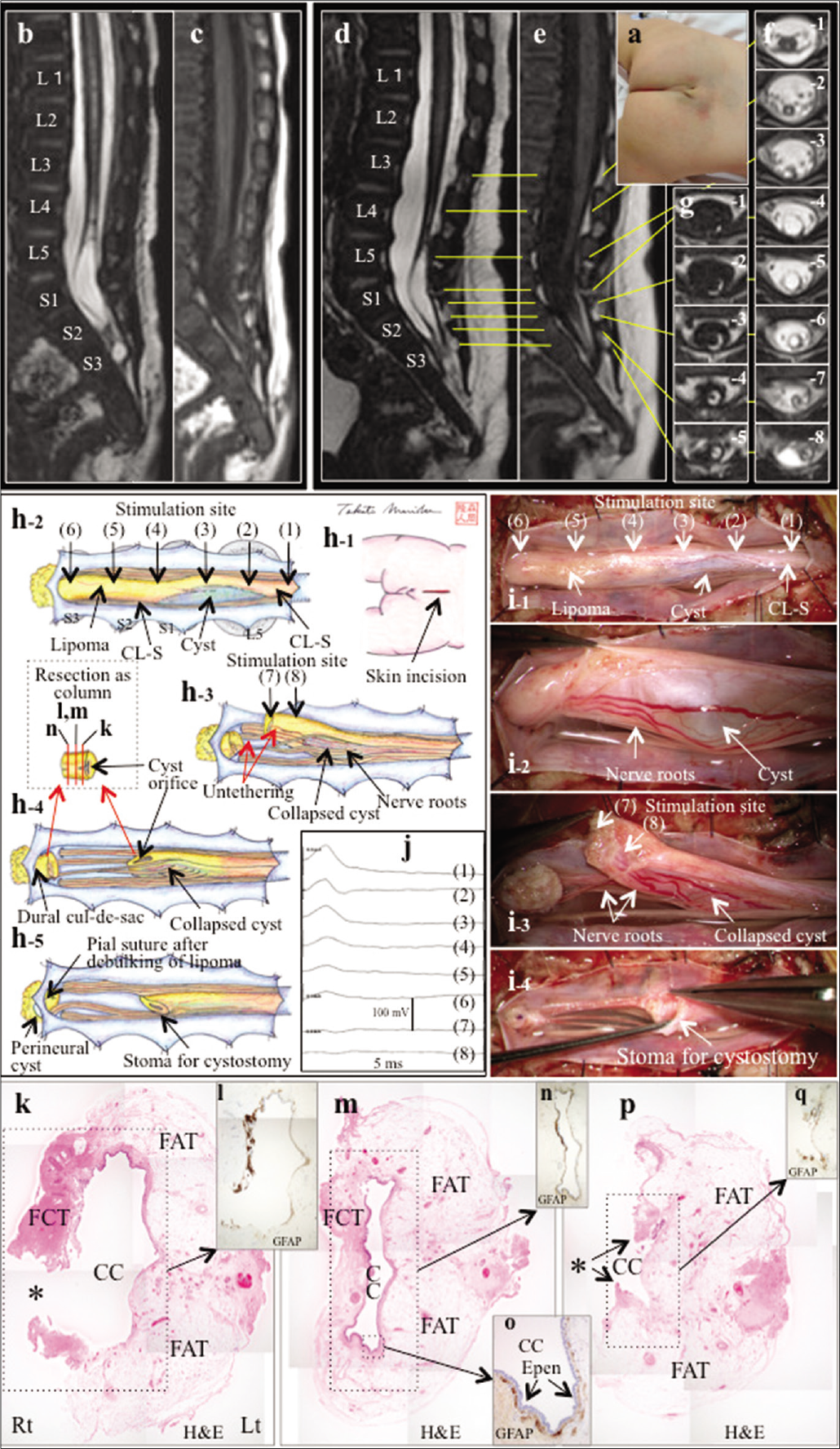
Patient 2 was a boy without neurological deficits. At birth, abnormal grooves continuous from the gluteal cleft were noted [
Figure 2:
(a) Photograph showing an abnormal Y-shaped groove and another groove, continuous from the gluteal cleft. (b-g) Preoperative images. Sagittal views (slice thickness of 1.25 mm) of 3D-hT2WI (b) and 3DT1WI (c) at 26 days of age, and sagittal views (slice thickness of 1.25 mm) of 3D-hT2WI (d) and 3DT1WI (e) and axial views (slice thickness of 3.9 mm) of T2WI (f) and T1-weighted image (g) at 3 months of age depict that the caudal spinal cord and continuous C-LS, with a large syringomyelia cyst at the L5-S2 level, extends to the dural culde-sac at the S2-3 level. The caudal and right half of the cyst wall is lipomatous tissue, which became more evident on the 2nd MRI. A sacral perineural cyst is also noted. (h-j) Schematic drawing (h) and microscopic view of the operative findings (i) and IONM (j). A lineal skin incision was made on the mid-lumbosacral region (h-1). Laminoplastic laminotomy of L5-S3 revealed that the caudal spinal cord and C-LS, including the cyst, extended to the dural cul-de-sac without an intervening terminal filum (h-2, i-1). The cyst wall and the lipomatous tissue were observed at the right and caudal side of the C-LS, respectively. IONM, by tracing the evoked CMAPs of the external anal sphincter with stimulation of 0.5 mA, failed to determine the border between the true cord and C-LS, while the amplitude of CMAPs tended to decrease toward the caudal side (j(1)-(6)), probably due to the current spread through the small nerve roots, which were tightly adhered to the ventral surface of the cord and C-LS (i-2). The lipoma with C-LS was severed at the caudal part and elevated (h-3). A small amount of water-like clear fluid flowed out from the cyst and the cyst was collapsed. After confirming nonfunctional C-LS and lipoma with IONM (j(7)(8)), the caudal part of the C-LS along with lipoma was resected as a column (h-4). The lipoma was minimally debulked to enlarge the cyst orifice and make a decent stoma for cystostomy (h-5, i-4). The caudal remnant lipoma was minimally debulked and the pial surface was reconstructed with sutures. No direct surgical procedure for sacral perineural cyst was performed. (k-q) Photomicrograph of cross-sections of the C-LS including lipoma with HE (k,m,p) and immunostaining for GFAP (l,n,o,q). The location of section is indicated as red line in (h-4) and the orientation is almost matched with that of (g-3,4). A lower or higher magnification view of the area is indicated by the dotted square in (k), (m), and (p). The C-LS, which consists of fibrocollagenous tissue embedding a central canal-like structure lined by ependymal cells and surrounded by GFAP-immunopositive neuroglial tissues, is located on the right side of the resected column. The lipoma, which consists of a mature fibroadipose tissue, is located on the left side of the column. Central canal-like structure decreased in size, moving caudally. *Indicates the destruction of the cyst wall created when preparing the specimen. Original magnification: (k-q) ×4 and (o) ×20.
Untethering surgery was performed at 4 months of age. Laminoplastic laminotomy of L5-S3 revealed that the caudal spinal cord and C-LS extended to the dural cul-desac [
The postoperative course was uneventful. Histopathological examination revealed that the C-LS was located on the right side of the resected column [
DISCUSSION
Although the exact anatomical relationship between RMC and the associated terminal lipoma was not demonstrated, Kim et al.[
In the IONM method, stimulation in the rostrocaudal direction is recommended.[
In patient 1, neuroimaging and intraoperative findings showed that the nonfunctional C-LS, continuous from the cord, and the terminal lipoma ran on the left and right sides of the column, respectively. Histopathological examination confirmed that the central canal-like ependyma lined lumen with surrounding neuroglial and fibrocollagenous tissues was located on the left side of the column and the fibroadipose tissue on the right. Patient 2 had the same clinicopathological findings as the previously reported cases of cystic RMC with terminal lipoma.[
In this study, the RMC was located in the lateral part rather than at the center of the lipoma, as reported in a previous study.[
The present findings support the idea raised by Pang et al.[
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We thank Dr. Tadahisa Shono, Department of Neurosurgery, Harasanshin Hospital, for supporting our study. We would like to thank Editage (www.editage.com) for English language editing.
References
1. Kim KH, Lee JY, Wang KC. Secondary neurulation defects-1: Retained medullary cord. J Korean Neurosurg Soc. 2020. 63: 314-20
2. Morioka T, Hashiguchi K, Yoshida F, Nagata S, Miyagi Y, Mihara F. Dynamic morphological changes in lumbosacral lipoma during the first months of life revealed by constructive interference in steady-state (CISS) MR imaging. Child Nerve Syst. 2007. 23: 415-20
3. Morioka T, Murakami N, Kanata A, Tsukamoto E, Suzuki OS. Retained medullary cord with sacral subcutaneous meningocele and congenital dermal sinus. Childs Nerv Syst. 2020. 36: 423-7
4. Morota N, Ihara S, Ogiwara H. New classification of spinal lipomas based on embryonic stage. J Neurosurg Pediatr. 2017. 19: 428-39
5. Mukae N, Morioka T, Suzuki SO, Murakami N, Shimogawa T, Kanata A. Two cases of large filar cyst associated with terminal lipoma: Relationship with retained medullary cord. World Neurosurg. 2020. 142: 294-8
6. Murakami N, Morioka T, Shimogawa T, Hashiguchi K, Mukae N, Uchihashi K. Retained medullary cord extending to a sacral subcutaneous meningocele. Childs Nerv Syst. 2018. 34: 527-33
7. Murakami N, Morioka T, Shimogawa T, Mukae N, Inoha S, Sasaguri T. Ependyma-lined canal with surrounding neuroglial tissues in lumbosacral lipomatous malformations: Relationship with retained medullary cord. Pediatr Neurosurg. 2018. 53: 387-94
8. Pang D, Chong S, Wang KC, Di Rocco C, Pang D, Rutka JT.editors. Secondary neurulation defects-1: Thickened filum terminale, retained medullary cord. Textbook of Pediatric Neurosurgery. Switzerland: Springer; 2020. p.
9. Pang D, Zovickian J, Moes GS. Retained medullary cord in humans: Late arrest of secondary neurulation. Neurosurgery. 2011. 68: 1500-19
10. Sala F, Barone G, Tramontano V, Gallo P, Ghimenton C. Retained medullary cord confirmed by intraoperative neurophysiological mapping. Childs Nerv Syst. 2014. 30: 1287-91
11. Shirozu N, Morioka T, Inoha S, Imamoto N, Sasaguri T. Enlargement of sacral subcutaneous meningocele associated with retained medullary cord. Childs Nerv Syst. 2018. 34: 1785-90