- Department of Neurosurgery, Fukuoka University, Chikushi Hospital, Chikushino, Fukuoka, Japan.
- Stroke Center, Fukuoka University, Chikushi Hospital, Chikushino, Fukuoka, Japan.
Correspondence Address:
Kouhei Nii
Stroke Center, Fukuoka University, Chikushi Hospital, Chikushino, Fukuoka, Japan.
DOI:10.25259/SNI_792_2020
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Yusuke Morinaga1, Kouhei Nii1, Yusuke Takemura1, Hayatsura Hanada1, Kimiya Sakamoto1, Yoko Hirata1, Ritsurou Inoue1, Jun Tsugawa2, Satoshi Kimura2, Kanako Kurihara2, Yuji Tateishi2, Toshio Higashi2. Types of intraparenchymal hematoma as a predictor after revascularization in patients with anterior circulation acute ischemic stroke. 17-Mar-2021;12:102
How to cite this URL: Yusuke Morinaga1, Kouhei Nii1, Yusuke Takemura1, Hayatsura Hanada1, Kimiya Sakamoto1, Yoko Hirata1, Ritsurou Inoue1, Jun Tsugawa2, Satoshi Kimura2, Kanako Kurihara2, Yuji Tateishi2, Toshio Higashi2. Types of intraparenchymal hematoma as a predictor after revascularization in patients with anterior circulation acute ischemic stroke. 17-Mar-2021;12:102. Available from: https://surgicalneurologyint.com/surgicalint-articles/10649/
Abstract
Background: Intracranial hemorrhage after revascularization for acute ischemic stroke is associated with poor outcomes. Few reports have examined the relationship between parenchymal hematoma after revascularization and clinical outcomes. This retrospective study aimed to investigate the risk factors and clinical outcomes of parenchymal hematoma after revascularization for acute ischemic stroke.
Methods: Ninety-three patients underwent revascularization for anterior circulation acute ischemic stroke. Patient characteristics and clinical outcomes were compared between patients with and without post procedural parenchymal hematoma using the following parameters: age, sex, occlusion location, presence of atrial fibrillation, diffusion-weighted imaging-Alberta stroke program early computed tomography score (DWI-ASPECTS), National Institute of Health Stroke Scale (NIHSS) score, recombinant tissue plasminogen activator, thrombolysis in cerebral infarction > 2b, door-to-puncture time, onset-to-recanalization time, number of passes, and modified Rankin Scale scores.
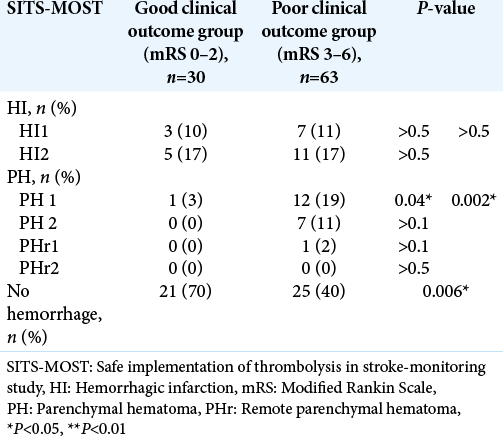
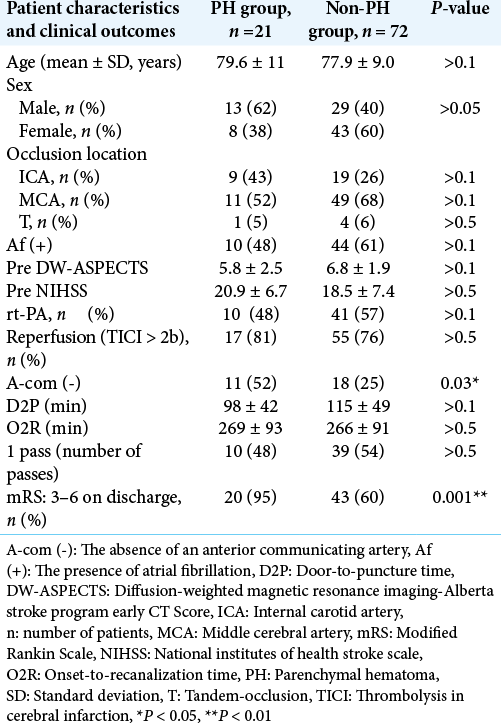
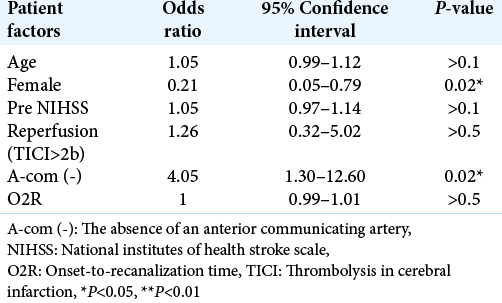
Results: Parenchymal hematomas were not significantly correlated with age, sex, occlusion location, atrial fibrillation, DWI-ASPECTS, NIHSS score, recombinant tissue plasminogen activator, thrombolysis in cerebral infarction > 2b, door-to-puncture time, onset-to-recanalization time, and number of passes, but were significantly correlated with poor clinical outcomes (P = 0.001) and absence of the anterior communicating artery evaluated using pre procedural time-of-flight magnetic resonance angiography (P = 0.03).
Conclusion: Parenchymal hematoma was a predictor of poor outcomes. In particular, the absence of the anterior communicating artery on pre procedural time-of-flight magnetic resonance angiography is a potential risk factor for parenchymal hematoma after revascularization for anterior circulation acute ischemic stroke.
Keywords: Cerebral hemorrhage, Cerebral revascularization, Cerebrovascular accident, Stroke, Thrombectomy
INTRODUCTION
Rapid, safe, and effective endovascular thrombectomy (EVT) to restore blood flow and ameliorate functional outcomes remain the primary goal of acute ischemic stroke (AIS) management,[
Collateral circulation is considered an important factor in determining the prognosis and response in recanalization therapy for AIS. Collateral circulation has been evaluated using computed tomography (CT) angiography, CT perfusion,[
The aim of this retrospective study was to investigate the risk factors, including A-com evaluated by TOF/MRA, and clinical outcomes of the hemorrhagic complication patterns after revascularization for anterior circulation AIS at our hospital.
MATERIALS AND METHODS
This single-center retrospective cohort study included 93 patients who underwent revascularization for anterior circulation AIS at our hospital between April 2014 and September 2019. We investigated on the anterior circulation lesions, including intracranial internal carotid artery (ICA), middle cerebral artery (MCA), and T occlusions.
All patients underwent EVT with a stent retriever and direct thromboaspiration. Recombinant tissue plasminogen activator (rt-PA) was administered before EVT in applicable cases, and an 8/9-French guiding balloon catheter was navigated into the ICA using the femoral approach. Thereafter, a microcatheter was passed through the occlusion site using a microguidewire through the aspiration catheter. The stent retriever, with a diameter of 4.0–6.0 mm, was retrieved during thromboaspiration with the aspiration catheter after temporary blockade of ICA flow. The postprocedural revascularization grade was evaluated by assigning the thrombolysis in cerebral infarction (TICI) grade.[
All patients underwent DW MRI before and after EVT as part of the evaluation for new ischemic lesions, and the presence or absence of the A-com was simultaneously recorded using TOF/MRA by two neurosurgeons (Y. M. and K. N.). In this study, the presence or absence of A-com is visually assessed by TOF/MRA signal intensity, which is a functional assessment of blood flow. The “presence” of A-com is defined when signal intensity is visually present on TOF/MRA, and the “absence” of A-com is defined when is absent. In other words, the absence of A-com includes the cases where the A-com is anatomically hypoplastic. Moreover, the DW Alberta Stroke Program Early CT Score (DW-ASPECTS) was used to assess the degree of the ischemia, and periprocedural neurologic findings were evaluated using the National Institutes of Health Stroke Scale (NIHSS).
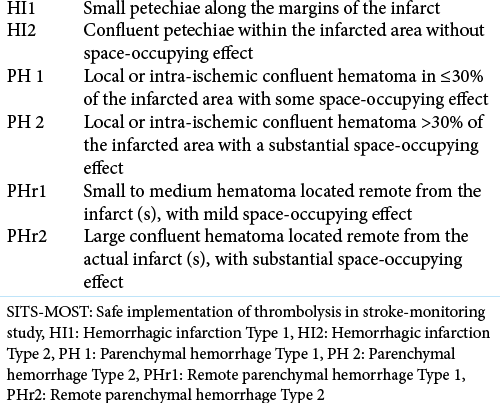
ICH was noted using CT within a day after EVT. The Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST) criteria were used to classify ICH [
First, univariate analysis was performed to determine any association between SITS-MOST and clinical outcomes. Subsequently, patient characteristics and clinical outcomes were compared between the parenchymal hematoma (PH) (n = 21) and non-PH groups (hemorrhagic infarction [HI] and no hemorrhage, n = 72), and the risk and prognostic factors were investigated. Predictors for PH were analyzed using multiple logistic regression analysis; the covariates were age, sex, NIHSS score, TICI grade, O2P, and absence of the A-com. Continuous variables are expressed as mean ± standard deviation, whereas categorical variables are expressed as percentages. Fisher’s exact test was used to analyze categorical variables, whereas the Mann–Whitney U-test was used for continuous variables. P < 0.05 was considered statistically significant. This study was conducted in accordance with the Declaration of Helsinki, and the study design was approved by the Fukuoka University Medical Ethics Committee (human subject assurance number: R19-002). Informed consent was obtained from the patients.
RESULTS
[
[
[
DISCUSSION
Revascularization using a stent retriever has increased the recanalization rates and resulted in good clinical outcomes in AIS with large vessel occlusion. However, the influence of overall ICH elicited by EVT requires further investigation. Kaesmacher et al.[
The PH subtype could also be a significant factor in the clinical outcomes of AIS treatment. It has been reported that the occurrence of both SICH and PH 2 after EVT leads to poor outcomes.[
Interestingly, our study showed that the absence of the A-com on pre procedural TOF-MRA was an independent risk factor of PH after EVT. Multiple logistic regression analyses revealed that the absence of the A-com significantly predicted PH [
Other previously reported risk factors of SICH or PH include the use of MERCI Retriever,[
The limitations of our study included the following: the choice of EVT devices depended on the site of the arterial occlusion and the preference of the surgeon. Our results require validation by future prospective studies that will not be limited by such selection bias. In addition, the 90-day outcome data were not available for analysis; this limited our ability to evaluate patient long-term outcomes. As rehabilitation often improves neurological assessments, we expect our rate of good clinical outcomes to have further increased after 90 days. This study enrolled a relatively small sample size; therefore, future studies with a larger number of cases should be conducted to confirm the present findings.
CONCLUSION
Our study showed that PH, not overall ICH, was a poor prognostic factor of clinical outcomes after revascularization in patients with anterior circulation AIS. In particular, the absence of the A-com on pre procedural TOF-MRA tended to be an independent risk factor for post-EVT PH. We consider that strict post procedural blood pressure control and careful imaging observations and administration of antithrombotics are important after EVT, especially when the A-com is absent, to prevent poor clinical outcomes caused by PH.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
References
1. Abou-Chebl A, Lin R, Hussain MS, Jovin TG, Levy EI, Liebeskind DS. Conscious sedation versus general anesthesia during endovascular therapy for acute anterior circulation stroke: Preliminary results from a retrospective, multicenter study. Stroke. 2010. 41: 1175-9
2. Bang OY, Saver JL, Kim SJ, Kim GM, Chung CS, Ovbiagele B. Collateral flow averts hemorrhagic transformation after endovascular therapy for acute ischemic stroke. Stroke. 2011. 42: 2235-9
3. Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015. 372: 11-20
4. Boisseau W, Fahed R, Lapergue B, Desilles JP, Zuber K, Khoury N. Predictors of parenchymal haematoma after mechanical thrombectomy: A multicenter study. Stroke. 2019. 50: 2364-70
5. Dorado L, Castaño C, Millán M, Aleu A, de la Ossa NP, Gomis M. Hemorrhagic risk of emergent endovascular treatment plus stenting in patients with acute ischemic stroke. J Stroke Cerebrovasc Dis. 2013. 22: 1326-31
6. Dzialowski I, Pexman JH, Barber PA, Demchuk AM, Buchan AM, Hill MD. Asymptomatic hemorrhage after thrombolysis may not be benign: Prognosis by hemorrhage type in the Canadian alteplase for stroke effectiveness study registry. Stroke. 2007. 38: 75-9
7. Evans MR, White P, Cowley P, Werring DJ. Revolution in acute ischaemic stroke care: A practical guide to mechanical thrombectomy. Pract Neurol. 2017. 17: 252-65
8. Fiorelli M, Bastianello S, von Kummer R, del Zoppo GJ, Larrue V, Lesaffre E. Hemorrhagic transformation within 36 hours of a cerebral infarct: Relationships with early clinical deterioration and 3-month outcome in the European cooperative acute stroke Study I (ECASS I) cohort. Stroke. 1999. 30: 2280-4
9. . Hemorrhage in the interventional management of stroke study. Stroke. 2006. 37: 847-51
10. Kaesmacher J, Kaesmacher M, Maegerlein C, Zimmer C, Gersing AS, Wunderlich S. Hemorrhagic transformations after thrombectomy: Risk factors and clinical relevance. Cerebrovasc Dis. 2007. 43: 294-304
11. Kono S, Deguchi K, Morimoto N, Kurara T, Deguchi S, Yamashita T. Tissue plasminogen activator thrombolytic therapy for acute ischemic stroke in 4 hospital groups in Japan. J Stroke Cerebrovasc Dis. 2016. 22: 190-6
12. Larrue V, von Kummer RR, Müller A, Bluhmki E. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: A secondary analysis of the European-Australasian acute stroke study (ECASS II). Stroke. 2001. 32: 438-41
13. Molina CA, Alvarez-Sabín J, Montaner J, Abilleira S, Arenillas JF, Coscojuela P. Thrombolysis-related hemorrhagic infarction: A marker of early reperfusion, reduced infarct size, and improved outcome in patients with proximal middle cerebral artery occlusion. Stroke. 2002. 33: 1551-6
14. Morinaga Y, Nii K, Sakamoto K, Inoue R, Mitsutake T, Hanada H. Presence of an anterior communicating artery as a prognostic factor in revascularization for anterior circulation acute ischemic stroke. World Neurosurg. 2019. 128: e660-3
15. Nogueira RG, Gupta R, Jovin TG, Levy EI, Liebeskind DS, Zaidat OO. Predictors and clinical relevance of hemorrhagic transformation after endovascular therapy for anterior circulation large vessel occlusion strokes: A multicenter retrospective analysis of 1122 patients. J Neurointerv Surg. 2017. 7: 16-21
16. Seyman E, Shaim H, Shenhar-Tsarfaty S, Jonash-Kimchi T, Bornstein NM, Hallevi H. The collateral circulation determines cortical infarct volume in anterior circulation ischemic stroke. BMC Neurol. 2016. 16: 206
17. Soize S, Barbe C, Kadziolka K, Estrade L, Serre I, Pierot L. Predictive factors of outcome and hemorrhage after acute ischemic stroke treated by mechanical thrombectomy with a stent-retriever. Neuroradiology. 2013. 55: 977-87
18. Tang B, Zeng J, Liu L, Xiao Y, Li Z, Zhang K. Evaluating the prognosis of ischemic stroke using low-dose multimodal computed tomography parameters in hyperacute phase. J Comput Assist Tomogr. 2017. 43: 22-8
19. Vora NA, Gupta R, Thomas AJ, Horowitz MB, Tayal AH, Hammer MD. Factors predicting hemorrhagic complications after multimodal reperfusion therapy for acute ischemic stroke. AJNR Am J Neuroradiol. 2007. 28: 1391-4
20. Wahlgren N, Ahmed N, Dávalos A, Ford GA, Grond M, Hacke W. Thrombolysis with alteplase for acute ischaemic stroke in the safe implementation of thrombolysis in stroke-monitoring study (SITS-MOST): An observational study. Lancet. 2007. 369: 275-82
21. Zhang S, Chen W, Tang H, Han Q, Yan S, Zhang X. The prognostic value of a four-dimensional CT angiography-based collateral grading scale for reperfusion therapy in acute ischemic stroke patients. PLoS One. 2016. 11: e0160502