- Department of Radiology University of Iowa, Iowa, United States.
- Department of Pathology, University of Iowa, Iowa, United States.
- Department of Radiology, University Texas Southwestern, Dallas, Texas, United States.
Correspondence Address:
Amit Agarwal
Department of Radiology, University Texas Southwestern, Dallas, Texas, United States.
DOI:10.25259/SNI_830_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Girish Bathla1, Sedat Giray Kandemirli1, Sarika Gupta2, Amit Agarwal3. Unique imaging appearance of neurosarcoidosis as a solitary cystic mass with mural enhancement. 22-Dec-2020;11:463
How to cite this URL: Girish Bathla1, Sedat Giray Kandemirli1, Sarika Gupta2, Amit Agarwal3. Unique imaging appearance of neurosarcoidosis as a solitary cystic mass with mural enhancement. 22-Dec-2020;11:463. Available from: https://surgicalneurologyint.com/surgicalint-articles/10488/
Abstract
Background: Sarcoidosis is an idiopathic, granulomatous, and multi-system inflammatory disorder that can also involve the central nervous system in the form of meningeal, parenchymal, or cranial nerve involvement. Imaging findings can be non-specific and may overlap with other inflammatory, infectious and neoplastic processes, and posing diagnostic challenges. Parenchymal involvement in neurosarcoidosis (NS) predominantly manifests as either non-enhancing white matter lesions or as enhancing parenchymal granulomas. Granulomas usually manifest as multiple solid lesions with nodular enhancement.
Case Description: A 72-year-old man presented with right-eye visual field changes with the non-contrast head computed tomography showing a large cystic lesion in the left frontoparietal lobe. Subsequent contrast-enhanced magnetic resonance imaging study revealed a large cystic mass with irregular rim enhancement and mural nodule concerning for glial neoplasm. Cyst decompression with biopsy and histopathological analysis revealed gliosis and prominent perivascular granulomatous inflammation with mixed picture of CD4 and CD8-positive cells suggestive of sarcoidosis. Further subsequent work-up showed mediastinal and cervical lymphadenopathy which on biopsy showed non-necrotizing granulomatous inflammation, consistent with sarcoidosis.
Conclusion: Herein, we report unique imaging findings of a NS case manifesting as a solitary cystic intraparenchymal lesion with an enhancing nodular component, mimicking primary intra-cranial tumor. This appearance is highly atypical and rarely been reported earlier.
Keywords: Cystic, Magnetic resonance imaging, Neurosarcoidosis
INTRODUCTION
Sarcoidosis is an idiopathic, granulomatous, multi-system inflammatory disorder, and characterized histologically by non-caseating granuloma formation. Sarcoidosis has a bimodal age distribution with initial peak in 3rd decade and a later peak after 50 years of age and a predilection for African Americans.[
Imaging findings in NS, however, are non-specific and may overlap with other inflammatory, infectious, and neoplastic processes. However, neurologic manifestations as initial clinical presenting symptom (seen in 52% of cases) and absence of systemic imaging at the time of CNS imaging can pose diagnostic challenges.[
Herein, we report unique imaging findings of a NS case manifesting as a solitary cystic intraparenchymal lesion with an enhancing nodular component, mimicking primary intracranial tumor. This appearance is highly atypical and rarely been reported earlier.
CASE REPORT
A 72-year-old man with a medical history of hypertension, glaucoma, and left retinal vein occlusion presented with right-eye visual field changes. Non-contrast head computed tomography showed a large intraparenchymal cystic lesion in the left frontoparietal region. Subsequent contrast-enhanced magnetic resonance imaging study revealed a large cystic mass lesion measuring 7.0 × 3.8 cm (transaxial) in the left frontoparietal lobe, following cerebrospinal fluid signal intensity on T1- and T2-weighted images with incomplete suppression of fluid signal fluid attenuation inversion recovery (FLAIR) sequence and no diffusion restriction [
Figure 1:
Axial fluid attenuation inversion recovery (FLAIR) (a) T2-weighted images (b) show a large cystic mass in the left frontoparietal region. Note incomplete suppression of fluid signal on FLAIR and mild perilesional edema and mass effect on the posterior horn of the left lateral ventricle. On post contrast image (c) there is complete rim enhancement and irregular, peripheral nodular enhancement in the anterior portion of the lesion. There is scalloping of the overlying bone cortex (arrow).
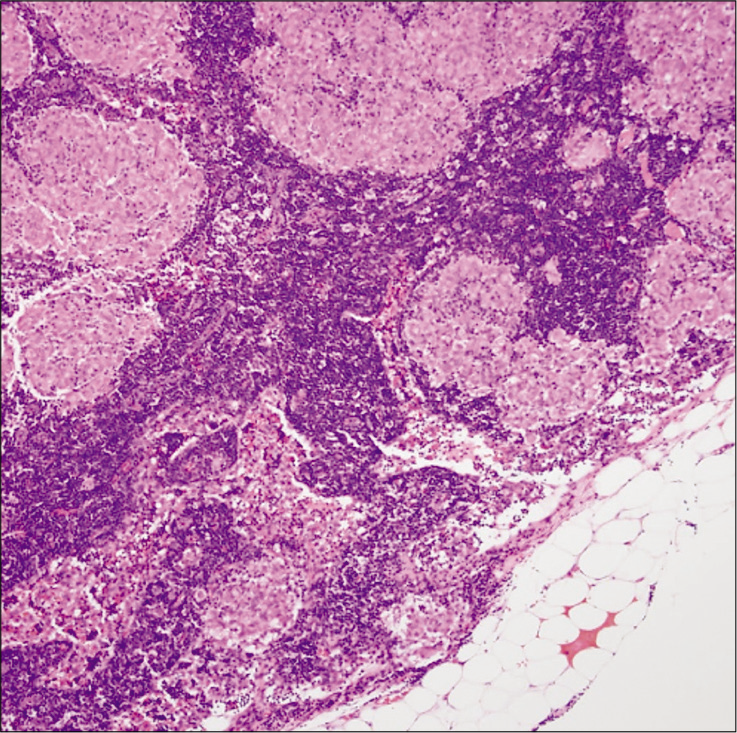
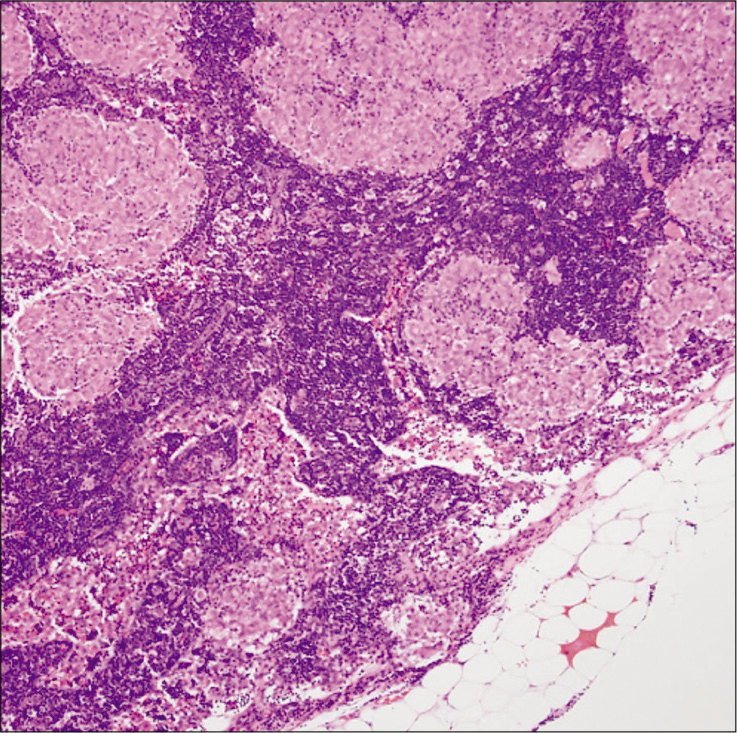
Histopathological analysis showed fragments of brain parenchyma with gliosis and prominent perivascular granulomatous inflammation with mixed picture of CD4 and CD8-positive cells [
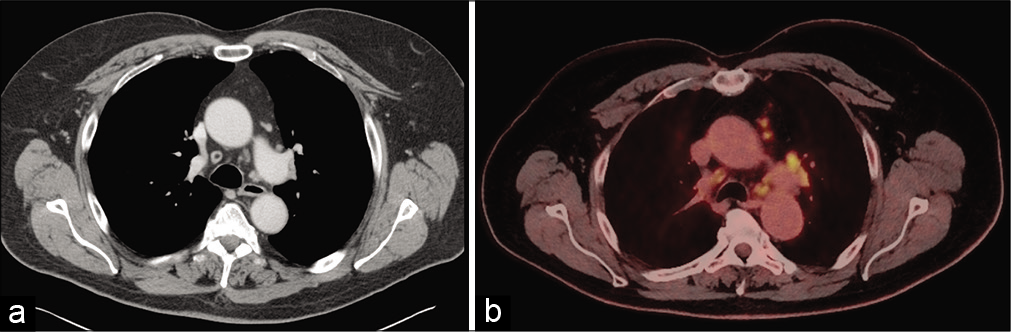
Further subsequent work-up including chest computed tomography and whole-body 2-[18F]-fluoro-2deoxy-Dglucose-positron emission tomography showed mediastinal and cervical lymphadenopathy [
DISCUSSION
Parenchymal involvement in NS may manifest as non-enhancing white matter lesions, enhancing granulomas, ischemic, or hemorrhagic lesions. The reported prevalence of parenchymal granulomas varies between 22% and 57%. Most of these lesions are supratentorial, sub-centimeter and show solid nodular enhancement on post contrast images.[
A review of the medical literature revealed only few case reports of NS with cystic parenchymal lesion. In the case reported by Al Hajri et al., there was a peripherally enhancing cystic lesion in the right temporal lobe.[
One of the plausible explanations for the granulomatous inflammation on pathology in the brain lesion could be a reactive granulomatous response to underlying neoplasm, as has been previously reported with seminomas.[
The mechanism of cystic degeneration in the current case remains uncertain. Ischemic necrosis or cystic degeneration of granuloma has been suggested as the cause of cyst formation in NS.[
Intracranial cysts and cystic-appearing masses have a broad imaging spectrum.[
In the current case, searching for underlying systemic sarcoidosis was helpful in confirming the diagnosis once the brain biopsy suggested NS. Fritz et al., in their meta-analysis, noted that neurological symptoms were the initial clinical manifestation in 52% of patients. In fact only 31% of patients had known sarcoidosis outside CNS at the time of diagnosis, even though 84% had systemic manifestations at any time during the course of the disease.[
CONCLUSION
We present a rare case of NS manifesting as an isolated large cystic lesion with enhancing mural nodule. Even though cystic degeneration may rarely occur in a sarcoid granuloma, it has not been previously described as an isolated finding. The presence of asymptomatic systemic sarcoidosis helped establish the correct diagnosis, given the highly atypical neuroimaging findings.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Al Hajri FA, Muqim AT, Muttikkal TJ. Neurosarcoidosis with intraparenchymal cystic lesions. A case report. Neuroradiol J. 2009. 21: 810-6
2. Bathla G, Singh AK, Policeni B, Agarwal A, Case B. Imaging of neurosarcoidosis: Common, uncommon, and rare. Clin Radiol. 2016. 71: 96-106
3. Fels C, Riegel A, Javaheripour-Otto K, Obenauer S. Neurosarcoidosis: Findings in MRI. Clin Imaging. 2004. 28: 166-9
4. Fritz D, van de Beek D, Brouwer MC. Clinical features, treatment and outcome in neurosarcoidosis: Systematic review and meta-analysis. BMC Neurol. 2016. 16: 220
5. Guoth MS, Kim J, de Lotbiniere AC, Brines ML. Neurosarcoidosis presenting as hypopituitarism and a cystic pituitary mass. Am J Med Sci. 1998. 315: 220-4
6. Nowak DA, Widenka DC. Neurosarcoidosis: A review of its intracranial manifestation. J Neurol. 2001. 248: 363-72
7. Oprisan A, Popescu BO. Intracranial cysts: An imagery diagnostic challenge. Sci World J. 2013. 2013: 172154
8. Peeples DM, Stern BJ, Jiji V, Sahni KS. Germ cell tumors masquerading as central nervous system sarcoidosis. Arch Neurol. 1991. 48: 554-6
9. Raz E, Zagzag D, Saba L, Mannelli L, di Paolo PL, D’Ambrosio F. Cyst with a mural nodule tumor of the brain. Cancer Imaging. 2012. 12: 237-44
10. Sato N, Sze G, Kim JH. Cystic pituitary mass in neurosarcoidosis. AJNR Am J Neuroradiol. 1997. 18: 1182-5
11. Shah R, Roberson GH, Cure JK. Correlation of MR imaging findings and clinical manifestations in neurosarcoidosis. AJNR Am J Neuroradiol. 2009. 30: 953-61
12. . Statement on sarcoidosis. joint statement of the American thoracic society (ATS), the European respiratory society (ERS) and the world association of sarcoidosis and other granulomatous disorders (WASOG) adopted by the ATS board of directors and by the ERS executive committee, February 1999. Am J Respir Crit Care Med. 1999. 160: 736-55
13. Utsuki S, Oka H, Suzuki S, Shimizu S, Tanizaki Y, Kondo K. Pathological and clinical features of cystic and noncystic glioblastomas. Brain Tumor Pathol. 2006. 23: 29-34