- Department of Neurosurgery, Japanese Red Cross Aichi Medical Center Nagoya Daiichi Hospital, Nagoya, Japan
- Department of Neurosurgery, Nagoya University Graduate School of Medicine, Nagoya, Japan.
Correspondence Address:
Takafumi Tanei, Department of Neurosurgery, Nagoya University Graduate School of Medicine, Nagoya, Japan.
DOI:10.25259/SNI_122_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Kentaro Wada1, Takafumi Tanei2, Kenichi Hattori1, Hisashi Hatano1, Shigeru Fujitani1, Risa Ito1, Hiroaki Kubo1, Yusuke Nishimura2, Satoshi Maesawa2, Ryuta Saito2. Unique vascular structures of a radicular arteriovenous fistula at the craniocervical junction along the first cervical spinal nerve: A case report. 10-Mar-2023;14:85
How to cite this URL: Kentaro Wada1, Takafumi Tanei2, Kenichi Hattori1, Hisashi Hatano1, Shigeru Fujitani1, Risa Ito1, Hiroaki Kubo1, Yusuke Nishimura2, Satoshi Maesawa2, Ryuta Saito2. Unique vascular structures of a radicular arteriovenous fistula at the craniocervical junction along the first cervical spinal nerve: A case report. 10-Mar-2023;14:85. Available from: https://surgicalneurologyint.com/surgicalint-articles/12184/
Abstract
Background: An arteriovenous fistula (AVF) at the craniocervical junction (CCJ) is a rare vascular malformation. Definitive diagnosis and curative treatment of CCJ AVF are challenging.
Case Description: A 77-year-old man presented with subarachnoid hemorrhage. Cerebral angiography showed an AVF at the CCJ, which drained into a radicular vein. The lesion was fed by a vertebral artery, anterior and lateral spinal arteries (LSAs), and the occipital artery (OA). There were two unique structures: the LSA originating from the posterior inferior cerebellar artery of the extracranial V3 segment and the OA feeding the shunt. Curative treatment involved two steps: endovascular embolization of feeders using Onyx and surgical shunt disconnection. Feeding arteries were blackened by Onyx, which helped identify the location of the shunt. The shunt was located behind the first cervical (C1) spinal nerve, and the draining vein was confirmed on the deep side of the nerve. A clip was applied to the draining vein distal to the shunt. Tiny vessels supplying the shunt were then coagulated referring to blackened arteries.
Conclusion: A radicular AVF at the CCJ along the C1 spinal nerve had unique vascular structures. Definitive diagnosis and curative treatment were achieved by combining endovascular embolization using Onyx and direct surgery.
Keywords: Craniocervical junction, Direct surgery, First cervical spinal nerve, Onyx, Radicular arteriovenous fistula
INTRODUCTION
Arteriovenous fistulas (AVFs) at the craniocervical junction (CCJ) are rare vascular malformations, accounting for approximately 2% of spinal AVFs.[
A case of a radicular AVF at the CCJ along the C1 spinal nerve with unique vascular structures is described. Definitive diagnosis and curative treatment were achieved by combining endovascular embolization using Onyx and direct surgery.
CASE DESCRIPTION
A 77-year-old man presented with sudden-onset headache and consciousness disturbance. His Glasgow coma scale was E3V4M5. Computed tomography (CT) showed massive subarachnoid hemorrhage in the posterior cranial fossa, especially around the medulla [
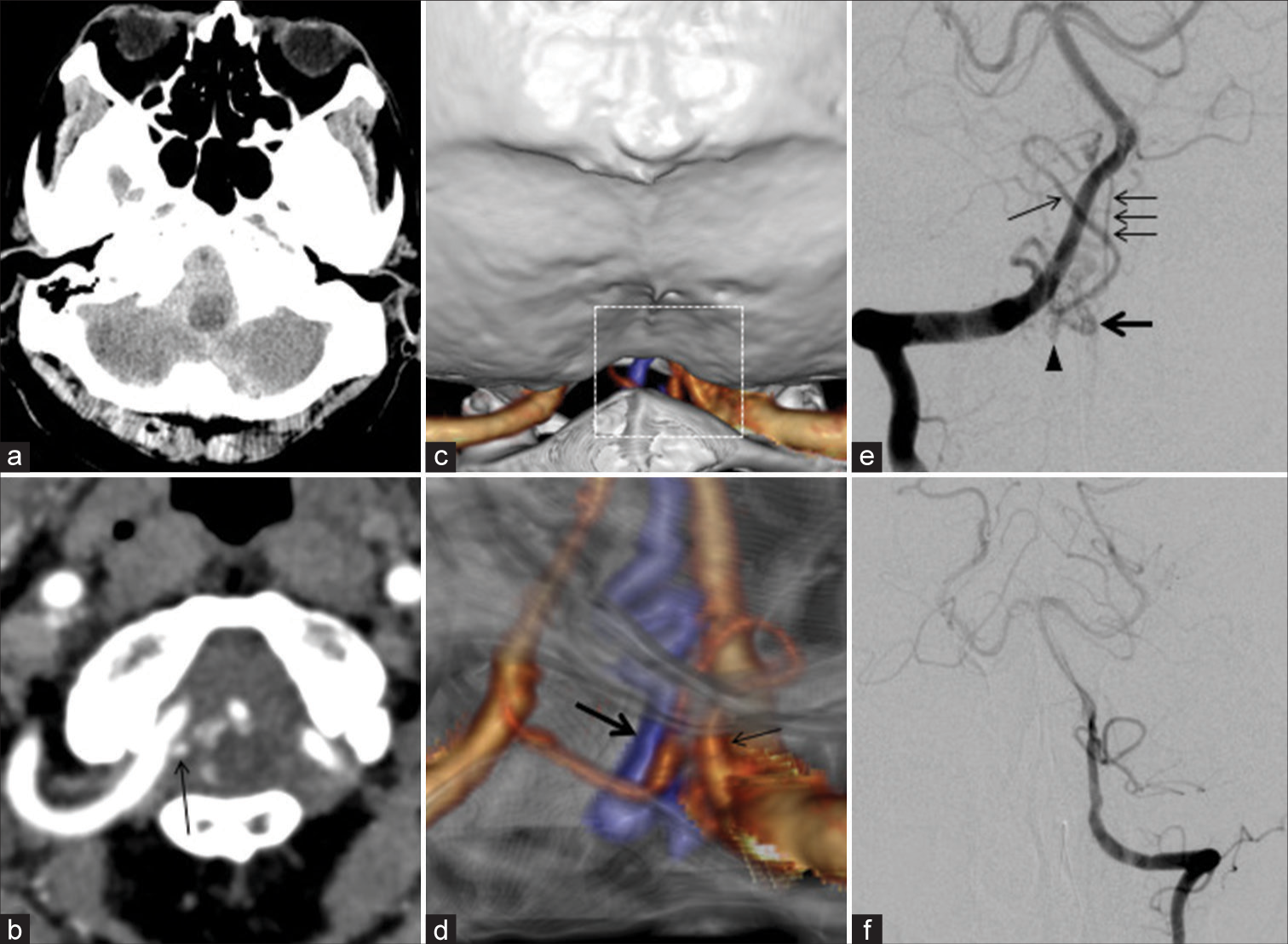
Figure 1:
Computed tomography shows subarachnoid hemorrhage in the posterior cranial fossa (a). Computed tomography angiography shows abnormal blood vessels around the right vertebral artery (VA) at the C1 level (b-d). d represents an enlargement of the white dot square part of c. Right VA angiography shows an arteriovenous fistula draining into a radicular vein (e). Left VA angiography does not demonstrate vascular lesions (f). (Arrow: Right posterior inferior cerebellar artery, Thick arrow: Draining vein, Arrowhead: Shunt, Arrows: Anterior spinal artery).
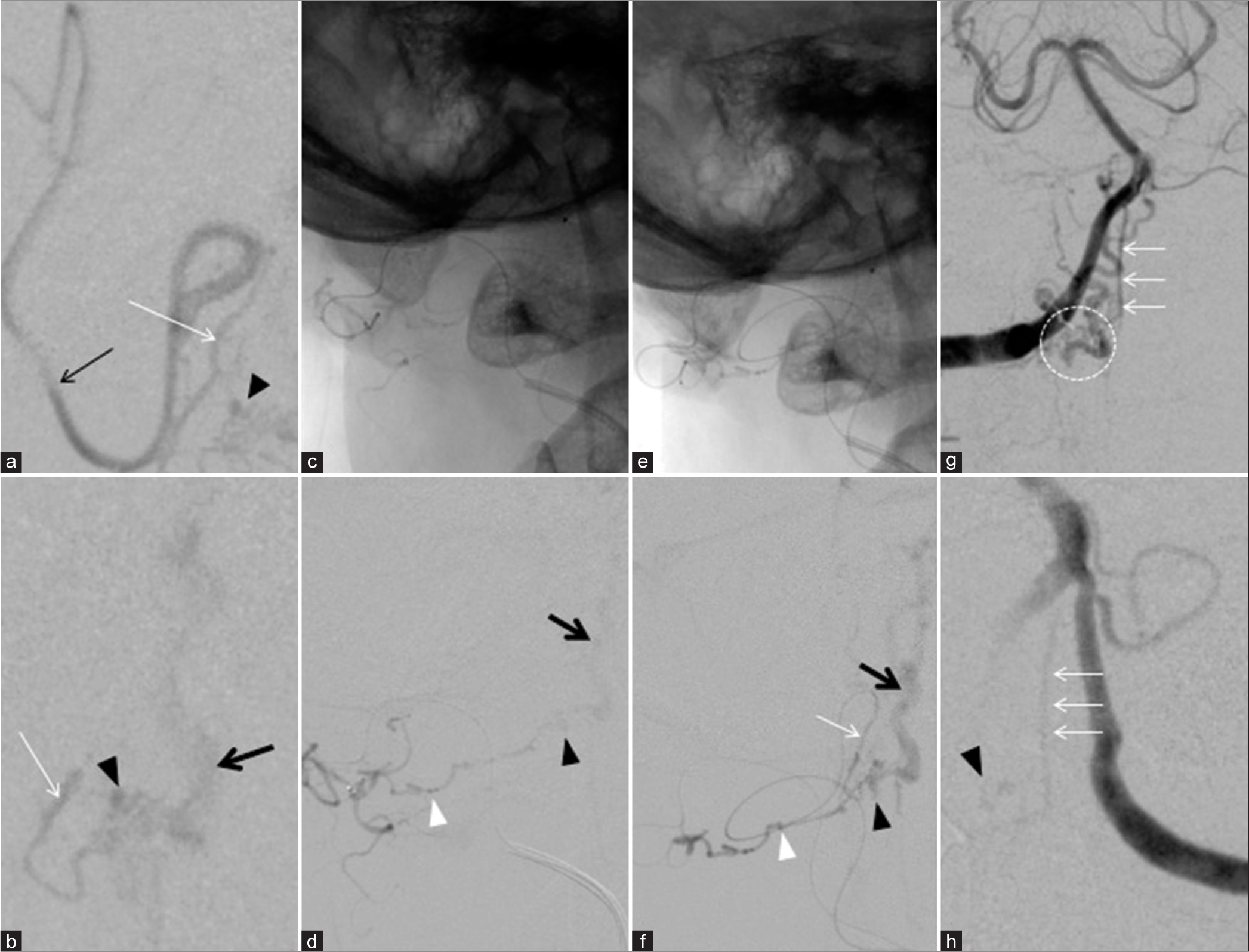
Figure 2:
Super-selective angiography shows that the right lateral spinal artery (LSA) originates from the right posterior inferior cerebellar artery and as a feeder (a and b). Super-selective angiography shows the occipital artery (OA) as a feeder (c and d). Super-selective angiography of both the LSA and the OA demonstrates each feeder supplying the same shunt (e and f). After embolization, right vertebral artery (VA) angiography shows residual flows from the right VA and the anterior spinal artery (ASA) (g). Left VA angiography also demonstrates the residual flow from the ASA (h). (White arrow: LSA, White arrows: ASA, White arrowhead: OA, Arrow: Posterior inferior cerebellar artery, Arrowhead: shunt, Thick arrow: Draining vein, Dot circle: Radiculomeningeal arteries from the right VA).
Endovascular embolization of the LSA and the OA feeders was performed using Onyx under general anesthesia. After embolization, the right VA angiography showed residual feeder flows from radiculomeningeal arteries of the right VA and spinal pial arteries of the ASA [
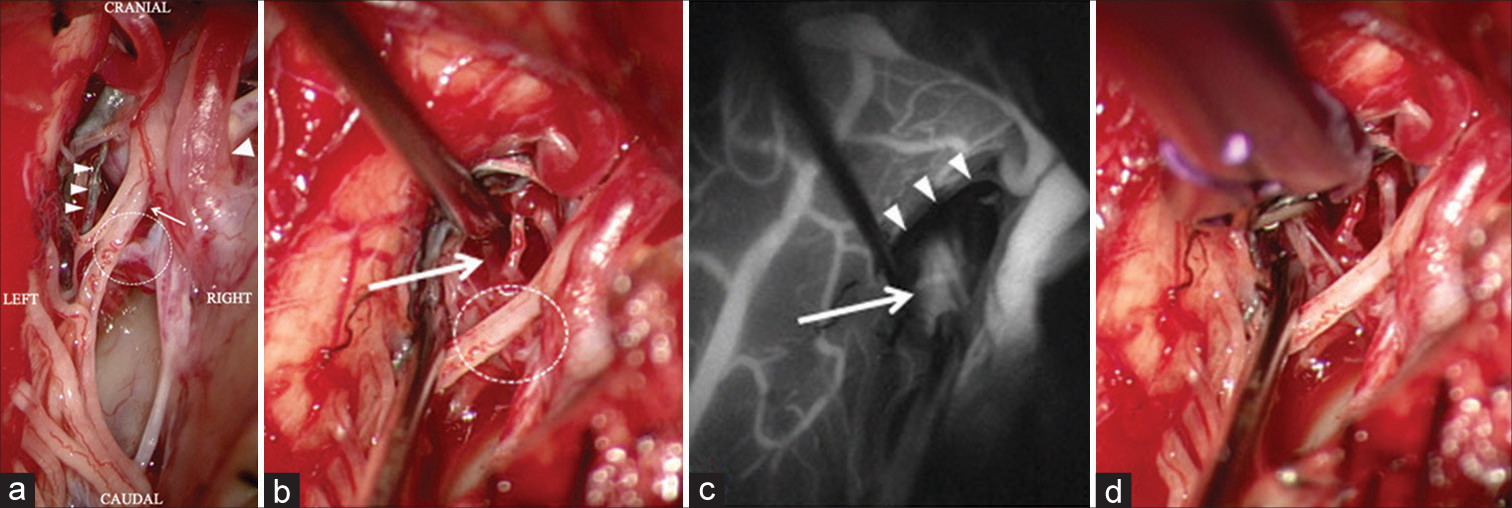
Figure 3:
Intraoperative photographs show the posterior inferior cerebellar artery penetrating the dura mater, the lateral spinal artery (LSA) is blackened by Onyx, and the shunt is located behind the first cervical (C1) spinal nerve (a). Another intraoperative photograph from a different angle shows a draining vein at the cranial deep side of the C1 spinal nerve (b). Intraoperative indocyanine green angiography shows that the draining vein is visible in the arterial phase (c). A clip is applied to the draining vein distal to the shunt (d). (Arrow: C1 spinal nerve, Arrowhead: Posterior inferior cerebellar artery, Arrowheads: LSA blackened by Onyx, Dot circle: Location of the shunt, Thick arrow: Draining vein).
DISCUSSION
CCF AVFs were once described as dural AVFs in the 1990s.[
Cerebral angiography and rotational CT angiography are the gold standard for diagnosis and can demonstrate small feeding arteries and venous varices.[
In the present case, the radicular AVF at the CCJ along the C1 spinal nerve had two unique vascular structures. The first was a feeder of the LSA originating from the PICA of the extracranial V3 segment. The unique structure of the PICA penetrating dura mater and the LSA originating from the proximal cranial loop of the PICA was confirmed by angiography and direct surgery. The PICA usually originates at the intracranial V4 segment, although about 5% to 20% originate at the extracranial V3 segment.[
Early treatment after initial hemorrhage will provide better outcomes, because the rebleeding rate of CCJ AVFs was 8.3%.[
Curative treatment of CCJ AVFs involves disconnecting or interrupting the connection between the shunt and draining vein.[
CONCLUSION
A case of a radicular AVF at the CCJ along the C1 spinal nerve presenting with subarachnoid hemorrhage was described. The lesion had two unique vascular structures, a feeder of the LSA originating from the PICA of the extracranial V3 segment and the OA feeding the shunt. Definitive diagnosis and curative treatment were achieved by combining endovascular embolization and direct surgery. Preoperative injections of Onyx into the main feeders were effective in reducing blood flow and allowed clear visualization of the anatomical structures around the shunt and the draining vein during direct surgery.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
This research was funded by Japanese Red Cross Aichi Medical Center Nagoya Daiichi Hospital Research Grant NFRCH23-0005.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Hiramatsu M, Sugiu K, Hishikawa T, Tokunaga K, Date I, Kuwayama N. Epidemiology of dural arteriovenous fistula in Japan: Analysis of Japanese registry of neuroendovascular therapy (JR-NET2). Neurol Med Chir (Tokyo). 2014. 54: 63-71
2. Hiramatsu M, Sugiu K, Ishiguro T, Kiyosue H, Sato K, Takai K. Angioarchitecture of arteriovenous fistulas at the craniocervical junction: A multicenter cohort study of 54 patients. J Neurosurg. 2018. 128: 1839-49
3. Iampreechakul P, Wangtanaphat K, Wattanasen Y, Hangsapruek S, Lertbutsayanukul P, Siriwimonmas S. Dural arteriovenous fistula of the craniocervical junction along the first cervical nerve: A single-center experience and review of the literature. Clin Neurol Neurosurg. 2022. 28;224: 107548
4. Isaji T, Yasuda M, Kawaguchi R, Aoyama M, Niwa A, Nakura T. Posterior inferior cerebellar artery with an extradural origin from the V3 segment: High incidence on the nondominant vertebral artery. J Neurosurg Spine. 2018. 28: 154-9
5. Kim DJ, Willinsky R, Geibprasert S, Krings T, Wallace C, Gentili F. Angiographic characteristics and treatment of cervical spinal dural arteriovenous shunts. AJNR Am J Neuroradiol. 2010. 31: 1512-5
6. Kim MS. Developmental anomalies of the distal vertebral artery and posterior inferior cerebellar artery: Diagnosis by CT angiography and literature review. Surg Radiol Anat. 2016. 38: 997-1006
7. Kinouchi H, Mizoi K, Takahashi A, Nagamine Y, Koshu K, Yoshimoto T. Dural arteriovenous shunts at the craniocervical junction. J Neurosurg. 1998. 89: 755-61
8. Lasjaunias P, Vallee B, Person H, Ter Brugge K, Chiu M. The lateral spinal artery of the upper cervical spinal cord. Anatomy, normal variations, and angiographic aspects. J Neurosurg. 1985. 63: 235-41
9. Macchi V, Porzionato A, Guidolin D, Parenti A, De Caro R. Morphogenesis of the posterior inferior cerebellar artery with three-dimensional reconstruction of the late embryonic vertebrobasilar system. Surg Radiol Anat. 2005. 27: 56-60
10. Matsubara S, Toi H, Takai H, Miyazaki Y, Kinoshita K, Sunada Y. Variations and management for patients with craniocervical junction arteriovenous fistulas: Comparison of dural, radicular, and epidural arteriovenous fistulas. Surg Neurol Int. 2021. 12: 411
11. Sato K, Endo T, Niizuma K, Fujimura M, Inoue T, Shimizu H. Concurrent dural and perimedullary arteriovenous fistulas at the craniocervical junction: Case series with special reference to angioarchitecture. J Neurosurg. 2013. 118: 451-9
12. Siclari F, Burger IM, Fasel JH, Gailloud P. Developmental anatomy of the distal vertebral artery in relationship to variants of the posterior and lateral spinal arterial systems. AJNR Am J Neuroradiol. 2007. 28: 1185-90
13. Takai K, Endo T, Seki T, Inoue T, Koyanagi I, Mitsuhara T. Ischemic complications in the neurosurgical and endovascular treatments of craniocervical junction arteriovenous fistulas: A multicenter study. J Neurosurg. 2022. 137: 1776-85
14. Wang JY, Molenda J, Bydon A, Colby GP, Coon AL, Tamargo RJ. Natural history and treatment of craniocervical junction dural arteriovenous fistulas. J Clin Neurosci. 2015. 22: 1701-7
15. Zhao J, Xu F, Ren J, Manjila S, Bambakidis NC. Dural arteriovenous fistulas at the craniocervical junction: A systematic review. J Neurointerv Surg. 2016. 8: 648-53
16. Zhong W, Zhang J, Shen J, Su W, Wang D, Zhang P. Dural arteriovenous fistulas at the craniocervical junction: A series case report. World Neurosurg. 2019. 122: e700-12