- College of Medicine, Princess Nourah Bint Abdulrahman University, Riyadh, Saudi Arabia.
- Department of Neurosurgery, King Faisal Specialist Hospital and Research Center, Riyadh, Saudi Arabia.
Correspondence Address:
Dr. Maher Hassounah, Department of Neurosurgery, King Faisal Specialized Hospital and Research Center, Riyadh, Saudi Arabia.
DOI:10.25259/SNI_311_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Raghad Hany Salem1, Abdulaziz Oqalaa Almubarak2, Maher Hassounah2. Unusual vascular anatomical variant leads to spontaneous hemorrhage in a cavum septum pellucidum in an adolescent: A case report and literature review. 01-Jul-2022;13:276
How to cite this URL: Raghad Hany Salem1, Abdulaziz Oqalaa Almubarak2, Maher Hassounah2. Unusual vascular anatomical variant leads to spontaneous hemorrhage in a cavum septum pellucidum in an adolescent: A case report and literature review. 01-Jul-2022;13:276. Available from: https://surgicalneurologyint.com/surgicalint-articles/11697/
Abstract
Background: Hemorrhagic lesions of the septum pellucidum are rare and usually occur in neonates. They can be due to a number of etiologies. Here, we report a rare case of adolescent nontraumatic septum pellucidum hemorrhage with a review of literature.
Case Description: A 16-year-old girl presented with a 1-month history of gradual visual deterioration in the left eye, intermittent headache, and vomiting. Brain imaging showed hematoma located between the leaflets of the septum pellucidum with obstructive hydrocephalus. Transcallosal resection of interventricular mass was done. The patient was discharged with improved neurological symptoms; however, the left eye vision did not recover. Imaging demonstrated a unique anatomical variant in deep vascular structures.
Conclusion: Cavum septum pellucidum hemorrhage is rare in adults. Many theories were constructed to explain its etiology. Bleeding due a vascular anatomical variant was not previously encountered. Understanding the embryological origin and anatomical details are important for proper clinical assessment and management of these patients.
Keywords: Anterior septal vein, Cavum septum pellucidum, Septocaval hematoma, Spontaneous hemorrhage, Vascular anatomical variant
INTRODUCTION
Cavum septum pellucidum (CSP) is a cerebrospinal fluid-filled, midline cavity located between the leaflets of the septum pellucidum. Spontaneous hemorrhage of septum pellucidum is extremely rare in older children and adults but is repeatedly reported in early infancy due to the delicacy of their blood vessels, immature cerebrovascular reactivity, and autoregulatory mechanisms. Trauma due to a shear injury of the brain is a recognized cause of corpus callosum and septum pellucidum hemorrhage. Most nontraumatic adult cases can be attributed to a ruptured anterior communicating artery or distal anterior cerebral artery aneurysms, ruptured arteriovenous malformations (AVM), or intracranial tumors such as glioblastoma multiforme and central neurocytoma with bleeding tendency. Occasionally, virus-associated encephalitis with hemorrhagic fever can be associated with CSP bleeding. Risk factors that can predispose to CSP hematoma formation include hypertension, diabetes, liver disease, alcohol consumption, smoking, abnormal blood vessel’s structure, and bleeding disorders. Here, we report a rare case of nontraumatic septum pellucidum hemorrhage in a previously healthy 16-year-old patient, along with its possible causes of arterial and venous anatomical variation. Related literature is reviewed.
CASE REPORT
Patient information
A 16-year-old girl presented to a local ophthalmology clinic complaining primarily of gradual impaired vision in the left eye for 1 month associated with intermittent headache and vomiting. She was referred to our center after having a computed tomography (CT) brain as a case of intraventricular brain tumor.
Clinical findings
Physical examination revealed a healthy appearing female who was not in distress. Vital signs were within normal. Glasgow Coma Score was 15/15. Pupils were 3 mm each and reactive. Visual acuity was OD 20/70 and OS 20/50. The left visual field was constricted. Fundus examination revealed severe bilateral papilledema. There was partial left abducens nerve palsy. Rest of the neurological examination was unremarkable.
Diagnostic assessment
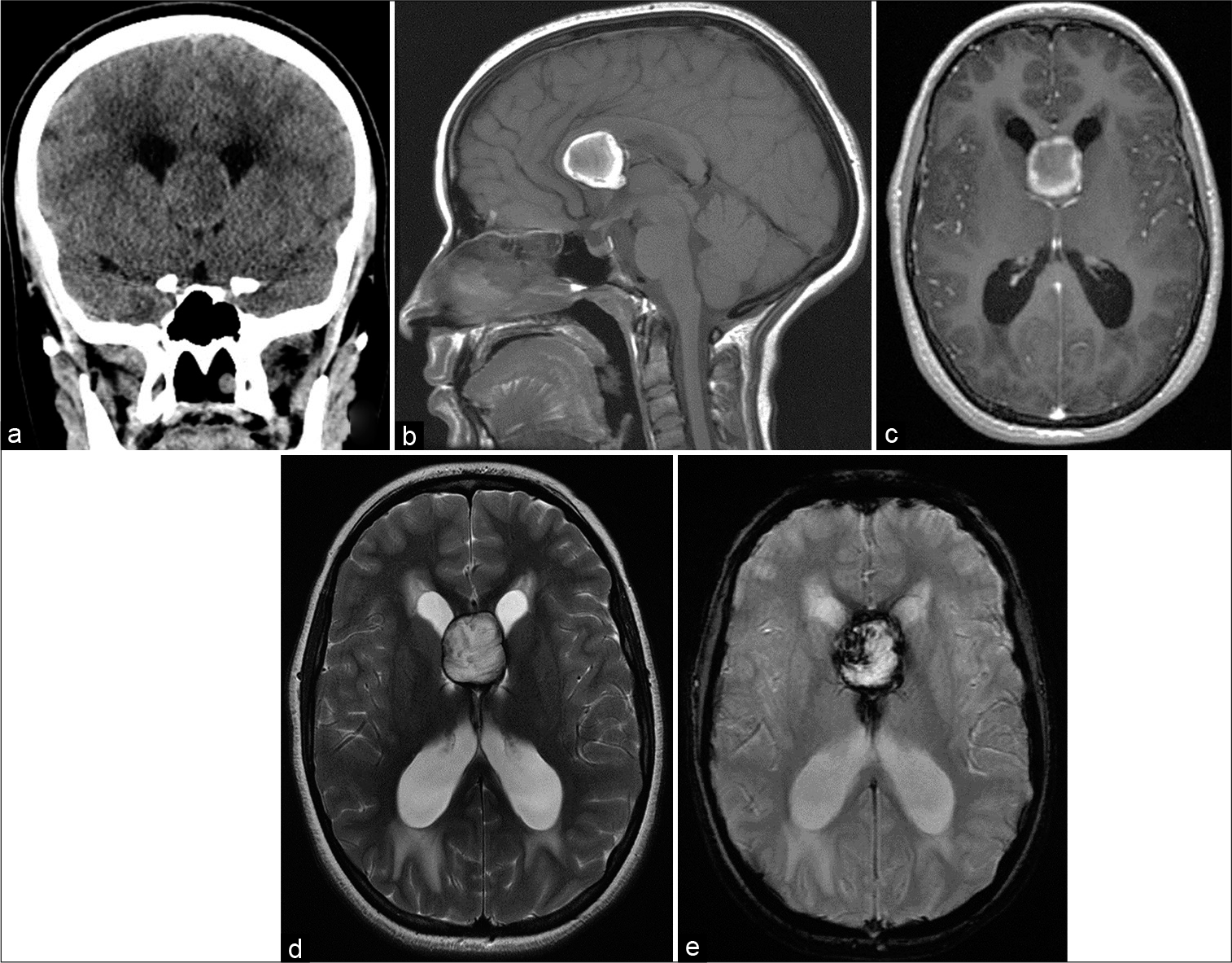
Complete blood count and coagulation profile were normal. CT of the brain showed anterior interventricular isodense lesion measuring 29 mm (anteroposterior) × 26 mm (transverse) × 27.5 mm (craniocaudal) [
Figure 1:
(a) Coronal computed tomography brain without contrast showing slightly hypodense septal lesion and hyperdense rim causing obstructive hydrocephalus with periventricular hypodensities. (b) Sagittal T1 magnetic resonance imaging (MRI) brain without contrast showing a hyperintense rim of the lesion. (c) Axial T1 MRI brain with contrast showing a nonenhancing interventricular mass within the cavum septum pellucidum. (d) Axial T2 MRI brain showing a thin dark rim of the lesion. (e) Susceptibility-weighted image showing blooming artifact.
Magnetic resonance imaging (MRI) of the brain showed a nonenhancing interventricular mass within the CSP exhibiting mixed signal intensities in both T1 and T2 with a thin dark rim signal on T2 and a blooming artifact on susceptibility-weighted image (SWI). The lateral ventricles were dilated with transependymal permeation [
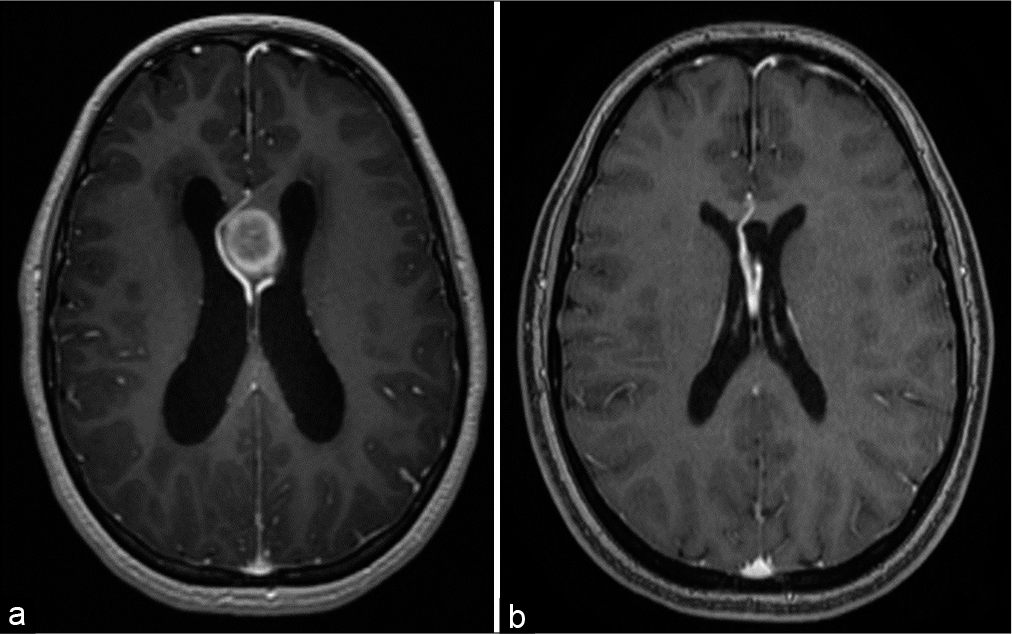
Magnetic resonance angiography (MRA) showed the right and left septal veins join and drain into an enlarged right internal cerebral vein (ICV). There was azygos anterior cerebral artery. The study did not reveal any aneurysm or AVM [
Figure 2:
(a) Magnetic resonance angiography (MRA) showing the right and left septal veins encasing the lesion and joining into an enlarged right internal cerebral vein. There is azygos anterior cerebral artery. (b) Postoperative MRA showing both septal veins. The right septal vein is very close to the azygos anterior cerebral artery.
Therapeutic intervention
The right frontal parasagittal craniotomy was performed. Through interhemispheric approach, the corpus callosum was opened and the encysted partially liquified hematoma was evacuated. The capsule of the hematoma which formed a cyst-like structure was dissected from the septum pellucidum leaflets and from a prominent right septal vein and was removed completely. There was no identifiable vascular lesion. Histopathological examination showed old hemorrhage (hemosiderin) and wall of a hemorrhagic cyst without lining cells or neoplastic cells. Her postoperative course was uneventful and her symptoms and neurological status improved apart from the left eye decreased vision.
Follow-up and outcome
The patient has been doing well during 7 years of follow-up. There have been no rebleeding episodes. MRI and MRA brain showed resolution of the obstructed hydrocephalus after eradicating the hematoma cyst. The previously mentioned venous anomaly continued to be observed [
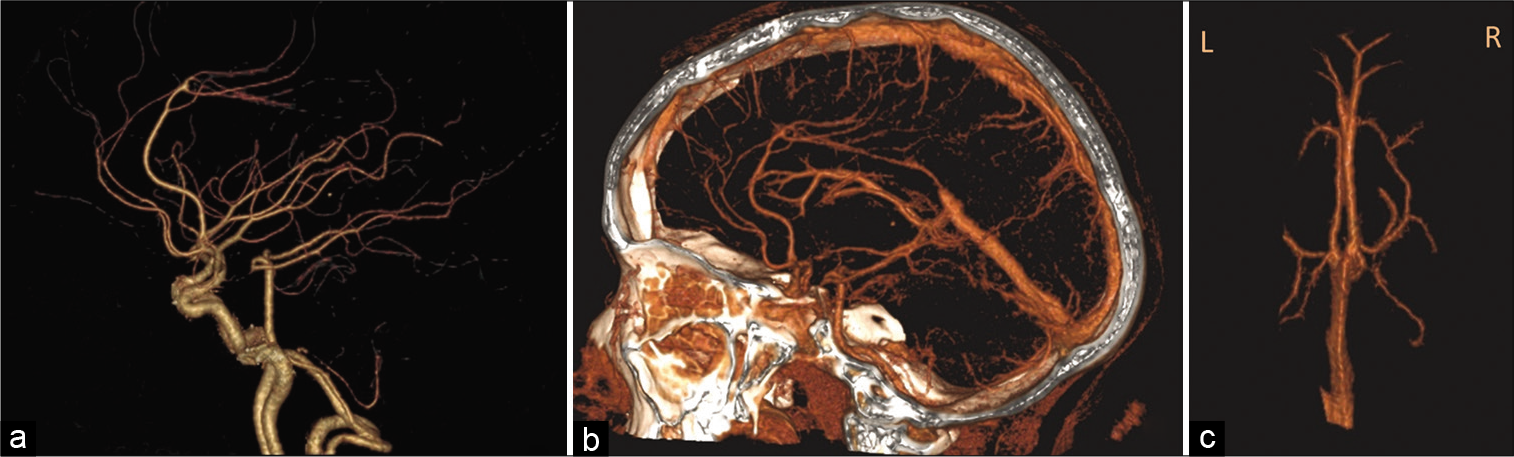
CT angiogram demonstrated several vascular anatomical variations. In the arterial phase, there was azygos pericallosal artery with an early origin of the callosomarginal arteries (triplicated anterior cerebral artery). There was a complex fenestration of the anterior communicating artery. No associated aneurysm or AVM was identified [
Figure 3:
(a) Computed tomography (CT) angiogram arterial phase, there is azygos pericallosal artery with an early origin of the callosomarginal arteries (triplicated anterior cerebral artery). (b) CT angiogram arterial and venous phases showing the close relation of the azygos artery and the septal veins. (c) CT angiogram venous phase, the anterior septal veins of both sides join, and drain into an enlarged right internal cerebral vein at the foramen of Monro where they are joined by the right thalamostriate vein. The small left internal cerebral vein receives only the left thalamostriate vein at the foramen of Monro.
In the venous phase, the anterior septal veins (ASVs) of both sides drained into an enlarged right ICV. The left septal vein looped inferiorly before rising to join its counterpart at the foramen of Monro where they were joined by the right thalamostriate vein (TSV). The small left ICV received only the left TSV at the foramen of Monro [
Conventional cerebral angiogram ruled out micro-AVM.
DISCUSSION
The CSP is an anatomical variant of septum pellucidum of the lateral ventricles. It is a midline structure that spans the space between the corpus callosum and the fornices. The walls of the CSP consist of white matter, fiber bundles, and is richly vascularized; hence, it is associated with the possibility of bleeding. The most common type of CSP is the noncommunicating CSP that is isolated from the ventricular system. Embryological development of the septum pellucidum is related to the corpus callosum. During early fetal development, the septum pellucidum develops a cavum by interstitial cleavage of commissural plate. About 85% of CSP shrink around 3–6 months after birth to a negligible slit; however, it may persist in around 20% of adults.
CSP hemorrhage is very uncommon, and the exact cause of the bleeding is debatable. The most common cause of CSP hematoma in adults is trauma.[
Conspicuously, when looking at literature discussing CSP bleeding, deep cerebral veins tend to be neglected in comparison to arteries. The ASV, as the name suggest, is responsible for draining the corpus callosum and deep medullary frontal white matter. They run on the anterior aspect of the anterior horns of the lateral ventricles then pass medially and inferior to the genu of the corpus callosum and terminate at the venous angle. The venous angle is an anatomic “U-shaped” landmark just posterior to the foramen of Monroe where the ICV meets its major tributary, the TSV, which mainly drains the basal ganglia. There is a paucity of literature on the variations of the ASV. The absence of a septal vein on one side was demonstrated in type 5 by Brzegowy et al. who classified variations of the ASV-ICV junction and its relation to the foramen of Monro into five types.[
Symptoms are nonspecific and usually related to increased intracranial pressure. Bleeding can either result in acute obstructive hydrocephalus or evolving hydrocephalus with gradual clinical deterioration because of progressive expansion of the hematoma, similar to this case, which gives the impression of underlying neoplastic or enlarging cystic lesions. Chronic encapsulated hematomas are known to form fibrous pseudocapsule with gradual expansion due to recurrent microhemorrhages into its core, mimicking neoplastic lesions.[
Due to the rare nature of this bleed in this particular location, there has not been a consensus in the literature regarding the ideal treatment modality. The majority of available documents on neonatal cases ended up with spontaneous quick resolution without any surgical intervention and no residual impairments. Management of septocaval hematomas in adults is directed to address increased ICP with definitive surgical treatment of the underlying cause to reduce the mass effect and verify the diagnosis. CSF shunting procedure might be required to relieve the hydrocephalus.
Repeated neurovascular studies and MRI on follow-up are very important to rule out underlying vascular or neoplastic pathologies.
CONCLUSION
CSP spontaneous hemorrhage is a rare clinical condition in adults. Most causes are attributed to underlying vascular anomaly or neoplasm. Bleeding due to vascular anatomical variation rather than vascular malformation has not been previously reported. Neurovascular and neuroimaging studies with long-term follow-up should be included in the management to recognize any distinct vascular variations and to rule out concealed small arteriovenous anomalies or tumors. More anatomical and radiological studies of the deep venous system are required.
Declaration of patient consent
Patients’ consent not required as patients’ identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to show our gratitude to Dr. Emad Alrasheed from radiology department for reviewing the images provided in this report.
References
1. Brzegowy K, Zarzecki M, Musiał A, Aziz HM, Kasprzycki T, Tubbs RS. The internal cerebral vein: New classification of branching patterns based on CTA. Am J Neuroradiol. 2019. 40: 1719-24
2. Erbaş G, Oner A, Akpek S, Tokgoz N. Corpus callosum hematoma secondary to isolated inferior sagittal sinus thrombosis. Acta Radiol. 2006. 47: 1085-8
3. Ghuman MS, Gupta V, Kumar A, Tiwari M, Khandelwal N. Hemorrhage in cavum septum pellucidum et vergae: It does exist!. Acta Neurochir. 2015. 157: 1489-91
4. Kanpolat Y, Mertol T. Haematoma of cavum septi pellucidi due to hypertension. Acta Neurochir. 1987. 89: 135-6
5. Kawahara I, Fujimoto T, Hirose M, Toba T. A rare case of subependymoma of the septum pellucidum as intratumoral hemorrhage. No Shinkei Geka. 2015. 43: 1105-11
6. Koumanidou C, Vakaki M, Anagnostara A, Pitsoulakis G, Kakavakis K, Mirilas P. Hemorrhage in the cavum septi pellucidi, and a brief review of the literature. Neuroradiology. 2002. 44: 770-4
7. Lindboe CF, Stolt-Nielsen A, Dale LG. Hemorrhage in a highly vascularized subependymoma of the septum pellucidum: Case report. Neurosurgery. 1992. 31: 741-5
8. Pinto JA, Melo JR, Pereira JR, Veiga-Pires JA. Haematomas of the septum pellucidum. Aetiology and incidence. Rofo. 1988. 148: 591-3
9. Roberti F, Bell J. Septum pellucidum chronic encapsulated hematoma with osseous metaplasia mimicking recurrent astrocytoma and shunt-related foreign body granuloma. Cureus. 2020. 12: e9839
10. Roditis S. Spontaneous hematoma of the septum pellucidum and corpus callosum: A case report. Rom Neurosurg. 2011. 18: 344-8
11. Sarwar M. The septum pellucidum: Normal and abnormal. Am J Neuroradiol. 1989. 10: 989-1005
12. Yanaka K, Meguro K, Narushima K, Takano S, Doi M, Nose T. Basal perforating artery aneurysm within the cavum septi pellucidi: Case report. J Neurosurg. 1998. 88: 601-4