- Department of Neurosurgery, Osaka Medical Pharmaceutical University Hospital, Takatsuki city, Japan.
Correspondence Address:
Naoki Omura, Department of Neurosurgery, Osaka Medical Pharmaceutical University Hospital, Takatsuki City, Japan.
DOI:10.25259/SNI_161_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Naoki Omura, Shinji Kawabata, Kohei Yoshimura, Ryokichi Yagi, Motomasa Furuse, Masahiko Wanibuchi. Using virtual lines of navigation for a successful transcortical approach. 12-May-2023;14:171
How to cite this URL: Naoki Omura, Shinji Kawabata, Kohei Yoshimura, Ryokichi Yagi, Motomasa Furuse, Masahiko Wanibuchi. Using virtual lines of navigation for a successful transcortical approach. 12-May-2023;14:171. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12319
Abstract
Background: Neuronavigation systems have become essential tools in image-guided neurosurgery that aid in the accurate resection of brain tumors. Recent advancements to these devices can indicate the precise location of lesions but can also project an augmented reality (AR) image on the microscope eyepiece to facilitate a successful surgical operation. Although the transcortical approach is a very popular method in neurosurgery, it can lead to disorientation and can cause unnecessary brain damage when the distance from the brain surface to the lesion is long. Herein, we report on an actual case in which a virtual line from AR images was used to assist the transcortical approach.
Methods: A virtual line connecting the entry point and the target point, which were set as the navigation route, was created using Stealth station S7® (Medtronic, Minneapolis, USA). This line was projected as an AR image on the microscope eyepiece. It was possible to reach the target point by proceeding through the white matter along the displayed virtual line.
Results: The lesion was reached within a short duration using virtual line without disorientation.
Conclusion: Setting a virtual line as an AR image using neuronavigation is a simple and accurate method that can effectively support the conventional transcortical approach.
Keywords: Augmented reality, Neuronavigation system, Transcortical approach, Virtual line
INTRODUCTION
Since the first reported use of a intraoperative frameless stereotactic navigation device by Roberts et al.,[
In neurosurgery, a transcortical approach, including the high parietal approach, is used for deeply located lesions, such as intraventricular tumors. However, this approach may lead to disorientation and can even cause unnecessary brain damage when the distance from the brain surface to the lesion is long. Although some techniques have been reported, which may prevent damage to the surrounding brain tissues,[
In our institution, we use a target-setting system that is installed in our neuronavigation system to display the approaching virtual route on the microscope eyepiece, and thus guide a virtual route to the lesion when performing a transcortical approach. Contrary to the conventional method of creating surgical images from preoperative images with a navigation system, this report presents a simple and easy method that displays surgical plans as virtual lines.
CASE PRESENTATION
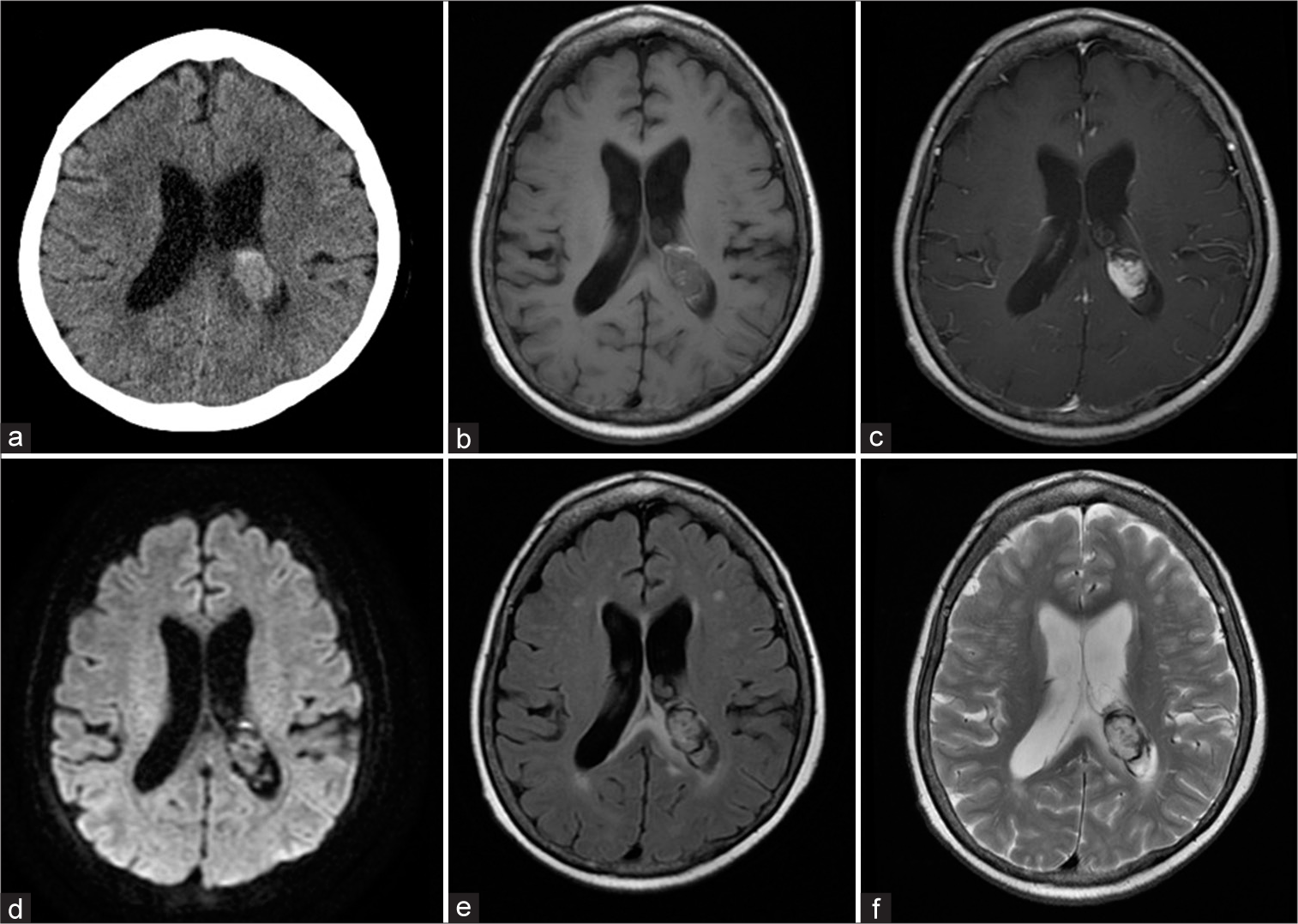
A 69-year-old female patient presented to an outpatient clinic with severe headache. The patient had a medical history of colon and breast cancer which had already been treated successfully. At the time of outpatient consultation, she only complained of headaches, and no neurological symptoms were observed. Computed tomography (CT) revealed hemorrhage from the posterior horn of the left-sided lateral ventricle to the trigone [
Figure 1:
Preoperative computed tomography (CT) and magnetic resonance images. (a). CT images revealed a mass lesion with bleeding in the posterior horn of the left ventricle to the trigone part. (b) T1-weighted, (c) enhanced T1-weighted, (d) diffusion-weighted imaging, (e) fluid-attenuated inversion recovery, and (f) T2-weighted images.
Surgical treatment
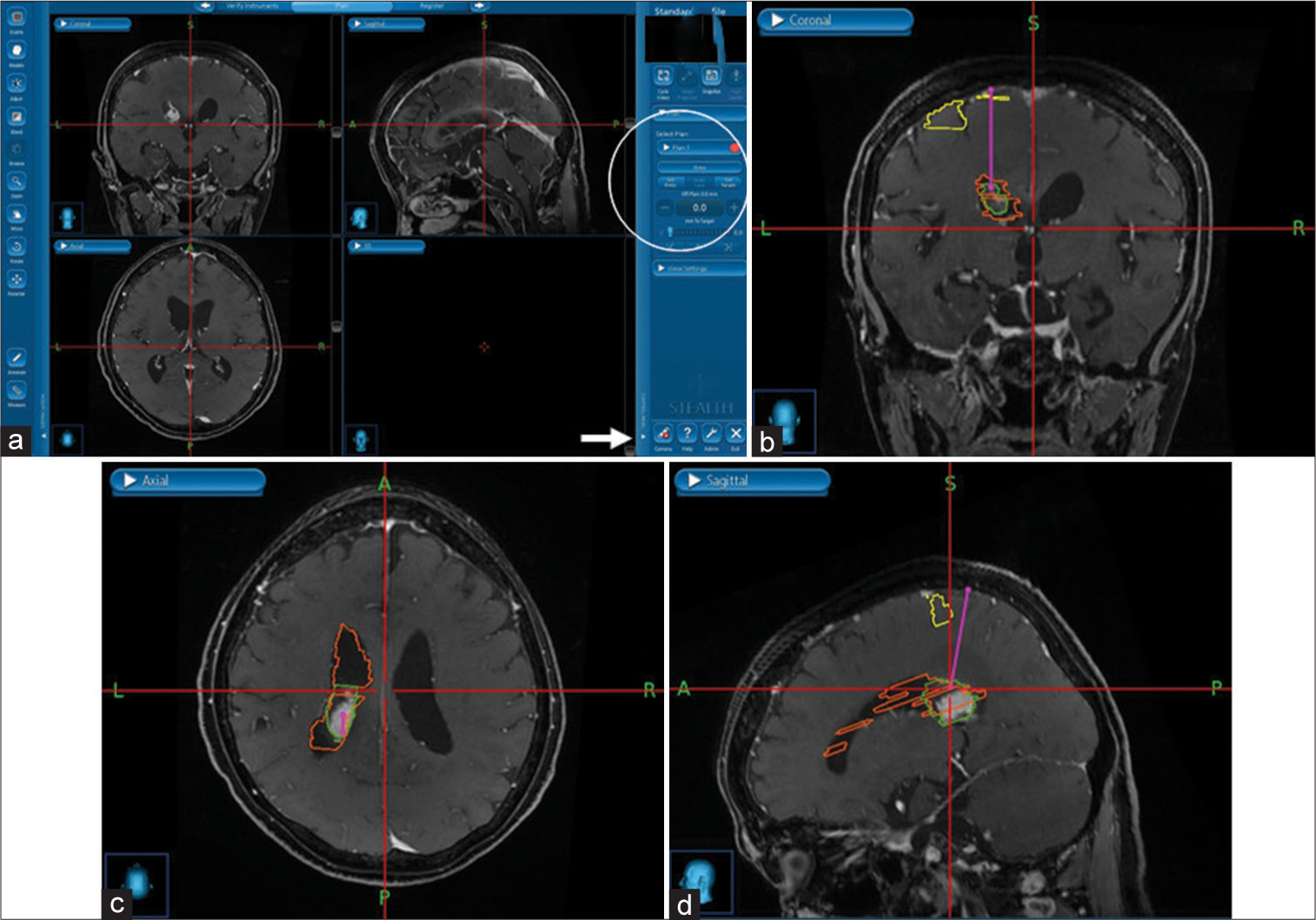
A high parietal approach was selected after considering the patient’s postoperative neurological complications. The patient underwent surgery in the prone position. In general, our institution uses the Stealth station S7® (Medtronic, Minneapolis, USA) as the intraoperative navigation device. Fusion of navigation information and intraoperative CT (SOMATOM Definition, Siemens, Munich, Germany) were employed for registration navigation. Next, areas of the postcentral gyrus, the left-sided lateral ventricle, and the mass lesion were extracted, which could be displayed as AR images on the microscope eyepiece. The approximate location of any marked structures could be confirmed from the surface of the patient’s skin before surgery by projecting AR images on the microscope eyepiece with this structure information. After craniotomy, the central sulcus and the postcentral gyrus were confirmed with a sensory evoked potential (SEP) of Neuromaster G1® (NIHON KOHDEN, Tokyo, Japan). Using navigation program for a needle biopsy in our Stealth station, the entry point was set at the point of entry superior parietal lobule, and the target point was set at the lesion in the left lateral ventricle. A line connecting these two points is drawn as the virtual line of the entry route [
Figure 2:
Surgical plan on the Stealth station. (a). When the tab displayed as plan on the right side of the screen was opened (arrow), items to set the entry and target were displayed (circle). (b-d). In this case, the superior parietal lobule was set as the entry, and the ventricular wall in contact with the mass lesion was set as the target. Consequently, a virtual line connecting these two points was displayed (pink line). The important structures during the operation were coloured to display the augmented reality image (post central gyrus was yellow; left ventricle was orange; mass lesion was green).
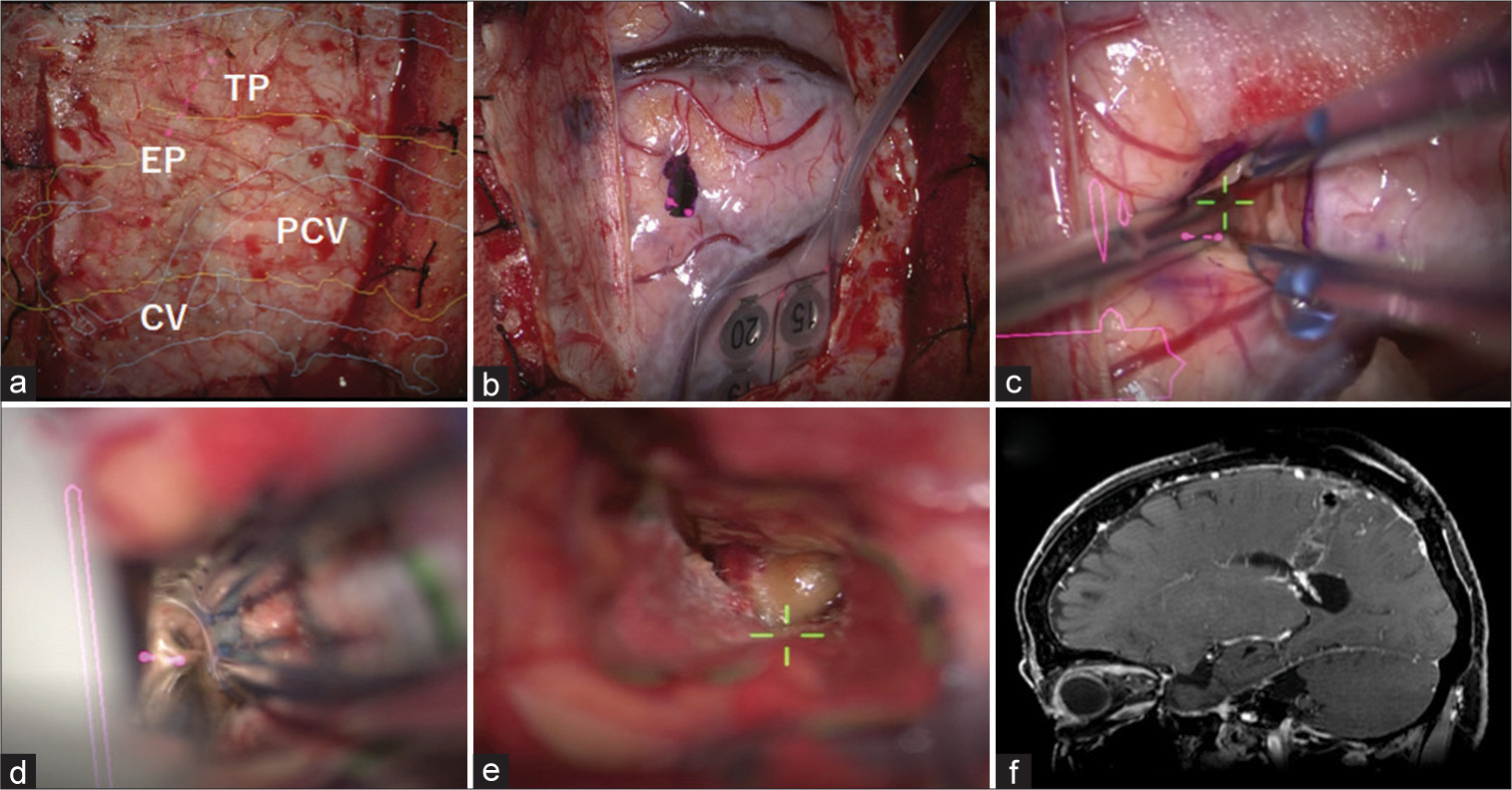
Figure 3:
Intraoperative and postoperative images. (a) After craniotomy, structures were confirmed with augmented reality images from the surface of the dura matter (PCV [yellow]: Postcentral gyrus, CV [blue]: cortical vein, EP: Entry point, and TP [pink dots]: Target point). The planned entry point was marked on the dura matter. (b) After opening the dura matter, the post central gyrus was confirmed with sensory evoked potential. The point to enter was marked. (c) After corticotomy at the entry point, the approach route was made along the virtual line (Central visual axis of the microscope was green mark). (d) Cerebral spinal fluid was found when reaching the ventricle. (e) A mass lesion with hemosiderin deposition was found in the ventricle (Green marks was central visual axis of the microscopic). (f) Postoperative magnetic resonance images showed total removal of the mass, and a tract which was in a straight line to the lesion.
Postoperation
The patient had mild left-sided sensory depression and agraphia after surgery. Postoperative MRI confirmed the successful total removal of the mass and a tract which was in a straight line to the lesion [
DISCUSSION
Since the introduction of the first intraoperative frameless stereotactic navigation device by Roberts et al., navigation systems in neurosurgery have become indispensable devices due to their ability to increase accuracy and safety.[
A transcortical approach is a commonly used technique in neurosurgery for intraparenchymal and intraventricular lesions. However, this approach may lead to disorientation and can even cause unnecessary brain damage when the distance from the brain surface to the lesion is long. In this study, we determined that the superior parietal approach is the most suitable approach for dominant-sided trigone lesion without neurological symptoms of approximately 20–25 mm to preserve the white fibers associated with speech and visual pathways. The incidence of neurological symptoms caused by this approach for trigone lesions has not yet been reported in detail, and it has been proposed that speech and cognitive deficits, if any, are mild and transient.[
Although some techniques have been reported which avoid damage to the surrounding white matter,[
Originally, the method shown in this case study uses the navigation program for a needle biopsy, where the tip of the navigation probe has been replaced by the surgeon’s eye. While there are reports where conventional use of AR images as surgical support is performed for orientation purposes by visualizing existing and avoiding important structures, the novel approach presented in this study is based on an ideal line which can assist surgery by visualizing the surgical approach as a virtual. Even though our navigation system is not up-to-date, this line can be created at any point in a very simple way and in a very short time, enabling accurate surgical processes through real-time assessment of this virtual line. Compared to needle biopsy, this method has the advantage in some cases that it uses fewer tools and can remove the tumor bulk. Recently, there have been several reports using the ViewSite Brain Access System (VBAS®; Vycor Medical Inc.) for intraventricular lesions.[
However, there are some important issues to note. The most important thing is that brain shift causes the navigation to be less accurate. Brain movement is associated with excessive cerebrospinal fluid loss, administration of mannitol, and hernia due to preoperative brain swelling.[
CONCLUSION
Setting a virtual line as an AR image is a simple and accurate method that can effectively support the conventional transcortical approach.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgments
The author would like to thank Toshihiro Takami for writing-review & Editing, Ryo Hiramatsu for formal analysis, and Masahiro Kameda and Naosuke Nonoguchi for software.
References
1. Akdemir H, Oktem S, Menkü A, Tucer B, Tuğcu B, Günaldi O. Image-guided microneurosurgical management of small arteriovenous malformation: Role of neuronavigation and intraoperative Doppler sonography. Minim Invasive Neurosurg. 2007. 50: 163-9
2. Bériault S, Sadikot AF, Alsubaie F, Drouin S, Collins DL, Pike GB. Neuronavigation using susceptibility-weighted venography: Application to deep brain stimulation and comparison with gadolinium contrast. J Neurosurg. 2014. 121: 131-41
3. Bertalanffy A, Roessler K, Koperek O, Gelpi E, Prayer D, Neuner M. Intraventricular meningiomas: A report of 16 cases. Neurosurg Rev. 2006. 29: 30-5
4. Bhatoe HS, Singh P, Dutta V. Intraventricular meningiomas: A clinicopathological study and review. Neurosurg Focus. 2006. 20: E9
5. Cho J, Rahimpour S, Cutler A, Goodwin CR, Lad SP, Codd P. Enhancing reality: A systematic review of augmented reality in neuronavigation and education. World Neurosurg. 2020. 139: 186-95
6. Cui ZQ, Ling ZP, Song HF, Hu S, Sun GC, Chen XL. Combining pyramidal tract mapping, microscopic-based neuronavigation, and intraoperative magnetic resonance imaging improves outcome of epilepsy foci resection in the sensorimotor cortex. Turk Neurosurg. 2014. 24: 538-45
7. Dasenbrock HH, See AP, Smalley RJ, Bi WL, Dolati P, Frerichs KU. Frameless stereotactic navigation during insular glioma resection using fusion of three-dimensional rotational angiography and magnetic resonance imaging. World Neurosurg. 2019. 126: 322-30
8. Gerard IJ, Hall JA, Mok K, Collins DL. New protocol for skin landmark registration in image-guided neurosurgery: Technical note. Neurosurgery. 2015. 11: 376-80 discussion 380
9. Gerard IJ, Kersten-Oertel M, Petrecca K, Sirhan D, Hall JA, Collins DL. Brain shift in neuronavigation of brain tumors: A review. Med Image Anal. 2017. 35: 403-20
10. Kersten-Oertel M, Gerard I, Drouin S, Mok K, Sirhan D, Sinclair DS. Augmented reality in neurovascular surgery: Feasibility and first uses in the operating room. Int J Comput Assist Radiol Surg. 2015. 10: 1823-36
11. Li B, Kim MG, Dominguez J, Feldstein E, Kleinman G, Hanft S. Intraventricular choroid plexus cavernoma resection using tubular retractor system and exoscope visualization: A technical case report. Oper Neurosurg ((Hagerstown). 2022. 22: e134-7
12. Muacevic A, Uhl E, Steiger HJ, Reulen HJ. Accuracy and clinical applicability of a passive marker based frameless neuronavigation system. J Clin Neurosci. 2000. 7: 414-8
13. Nimsky C, Ganslandt O, Fahlbusch R. Implementation of fiber tract navigation. Neurosurgery. 2006. 58: discussion ONS-303-4
14. Okasha M, Ineson G, Pesic-Smith J, Surash S. Transcortical approach to deep-seated intraventricular and intra-axial tumors using a tubular retractor system: A technical note and review of the literature. J Neurol Surg A Cent Eur Neurosurg. 2021. 82: 270-7
15. Passeri T, Giammattei L, Abbritti R, di Russo P, Bernat AL, Penet N. A new simple and free tubular device for microscopic transcortical approach to deep-seated lesions: Technical note and case example. Acta Neurochir (Acta). 2022. 164: 2049-55
16. Roberts DW, Strohbehn JW, Hatch JF, Murray W, Kettenberger H. A frameless stereotaxic integration of computerized tomographic imaging and the operating microscope. J Neurosurg. 1986. 65: 545-9
17. Satoh M, Nakajima T, Yamaguchi T, Watanabe E, Kawai K. Evaluation of augmented-reality based navigation for brain tumor surgery. J Clin Neurosci. 2021. 94: 305-14
18. Watanabe E, Satoh M, Konno T, Hirai M, Yamaguchi T. The trans-visible navigator: A see-through neuronavigation system using augmented reality. World Neurosurg. 2016. 87: 399-405