- Department of Neurosurgery, University of Miami Miller School of Medicine, Miami, Florida, United States.
- Department of Emergency, Jackson Memorial Hospital, Miami, Florida, United States.
- Department of Medicine, Jackson Memorial Hospital, Miami, Florida, United States.
- Department of Neurosurgery, University of Alabama Birmingham School of Medicine, Birmingham, Alabama, United States.
- Department of Orthopedic, Division of Neurology and Surgery, Jackson Memorial Hospital, Miami, Florida, United States.
- Department of Quality, Jackson Memorial Hospital, Miami, Florida, United States.
- Department of Nursing Education, Jackson Memorial Hospital, Miami, Florida, United States.
- Department of Neurology, University of Miami Miller School of Medicine, Miami, Florida, United States.
Correspondence Address:
Henry Chang, Department of Neurosurgery, University of Miami Miller School of Medicine, 1600 NW 10th Ave #1140, Miami - 33136, Florida, United States.
DOI:10.25259/SNI_371_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Henry Chang1, Michael Silva1, Alexander Giner2, Yvonne Diaz3, Marie Ann Sosa3, Grace Knudsen1, Anil K. Mahavadi4, June Ellis3, Arlene Cameron5, Carlos Andrei Quirós Núñez6, Millicent A. Wynter7, Kristine O’Phelan8, Ricardo J. Komotar1, Iahn Cajigas1. Ventriculostomy supply cart decreases time-to-external ventricular drain placement in the emergency department. 19-Jul-2021;12:362
How to cite this URL: Henry Chang1, Michael Silva1, Alexander Giner2, Yvonne Diaz3, Marie Ann Sosa3, Grace Knudsen1, Anil K. Mahavadi4, June Ellis3, Arlene Cameron5, Carlos Andrei Quirós Núñez6, Millicent A. Wynter7, Kristine O’Phelan8, Ricardo J. Komotar1, Iahn Cajigas1. Ventriculostomy supply cart decreases time-to-external ventricular drain placement in the emergency department. 19-Jul-2021;12:362. Available from: https://surgicalneurologyint.com/surgicalint-articles/ventriculostomy-supply-cart-decreases-time-to-external-ventricular-drain-placement-in-the-emergency-department/
Abstract
Background: Minimizing time-to-external ventricular drain (EVD) placement in the emergency department (ED) is critical. We sought to understand factors affecting time-to-EVD placement through a quality improvement initiative.
Methods: The use of process mapping, root cause analyses, and interviews with staff revealed decentralized supply storage as a major contributor to delays in EVD placement. We developed an EVD “crash cart” as a potential solution to this problem. Time-to-EVD placement was tracked prospectively using time stamps in the electronic medical record (EMR); precart control patients were reviewed retrospectively.
Results: The final cohorts consisted of 33 precart and 18 postcart cases. The mean time-to-EVD in the precart group was 99.09 min compared to 71.88 min in the postcart group (two-tailed t-test, P = 0.023). Median time-to-EVD was 92 min in the precart group compared to 64 min in the postcart group (rank sum test, P = 0.0165). Postcart patients trended toward improved outcomes with lower modified Rankin score scores at 1 year, but this did not reach statistical significance (two-tailed t-test, P = 0.177).
Conclusion: An EVD “crash cart” is a simple intervention that can significantly reduce time-to-EVD placement and may improve outcomes in patients requiring an EVD.
Keywords: Emergency department, Hydrocephalus, Quality improvement, Ventriculostomy
INTRODUCTION
Ventriculostomy placement is one of the most common neurosurgical bedside procedures. More than 20,000 external ventricular drains (EVDs) are placed annually in the United States.[
The majority of EVDs at our institution are placed in the ED, especially for patients with more urgent needs for EVD placement who require EVD placement before an ICU bed becomes available. Indications for emergent EVD placement in the ED are case specific, but typically include cases of aneurysmal subarachnoid hemorrhage with a Hunt and Hess grade of 3 or higher, acute hydrocephalus with a Glasgow Coma Scale (GCS) score of <15, intraventricular hemorrhage with hydrocephalus, and traumatic brain injuries that meet the Brain Trauma Foundation criteria for intracranial pressure (ICP) monitoring.[
MATERIALS AND METHODS
Study characteristics, inclusion/exclusion criteria
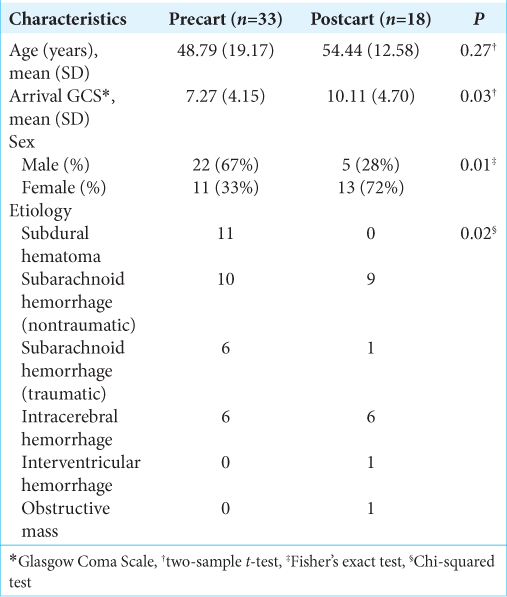
Data were collected both prospectively and retrospectively. The study was approved by our institution’s IRB (Protocol #20190342). All patient data were anonymized and unidentifiable throughout the data analysis process. We prospectively collected data on patients who underwent EVD placement in the ED after initiation of the EVD supply cart in the ED in January 2018. For our control group, we retrospectively reviewed patients who underwent EVD placement in the ED before implementation of the EVD supply cart. Patients were excluded from our analysis if there was a delay to EVD placement unrelated to the standard protocol (administration of blood products before EVD placement, reversal of decision to not place EVD based on delayed neurologic decline) or if accurate time stamps were not recorded in the electronic medical record (EMR) and complete EMRs were unable to be obtained. All patients that did not meet the exclusion criteria were included in the study. Cohort demographics (age, arrival, GCS, sex, and etiology) were collected and analyzed [
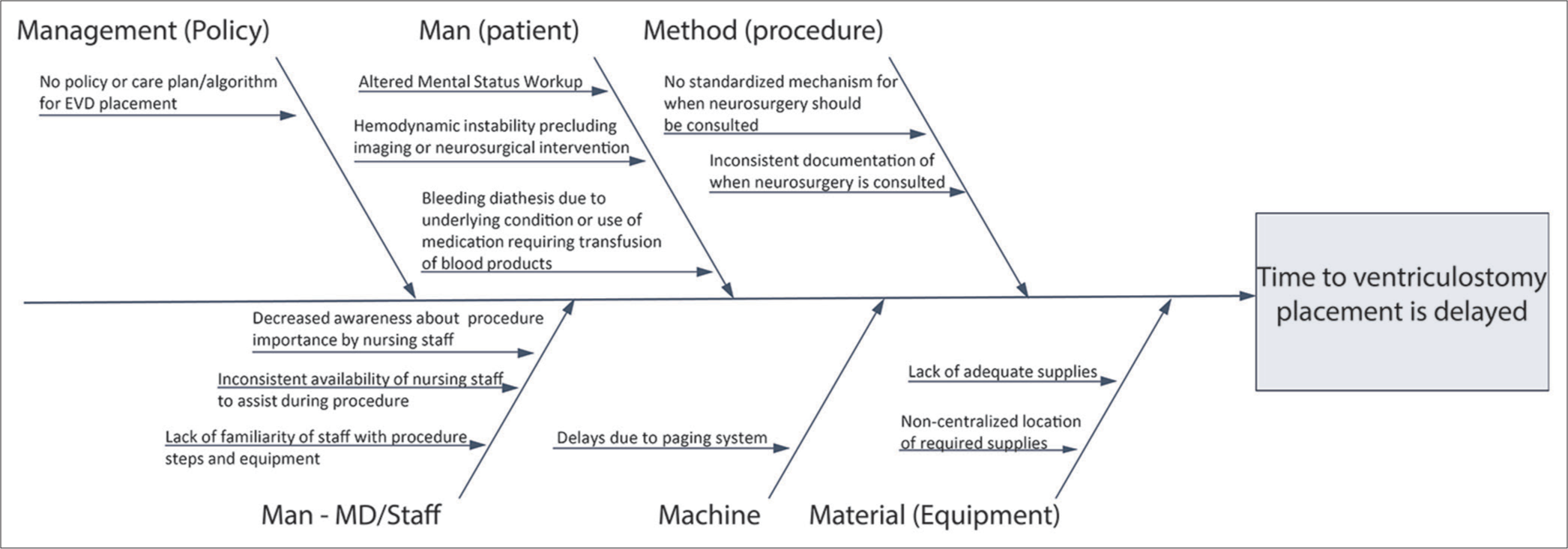
Root cause analysis and process mapping
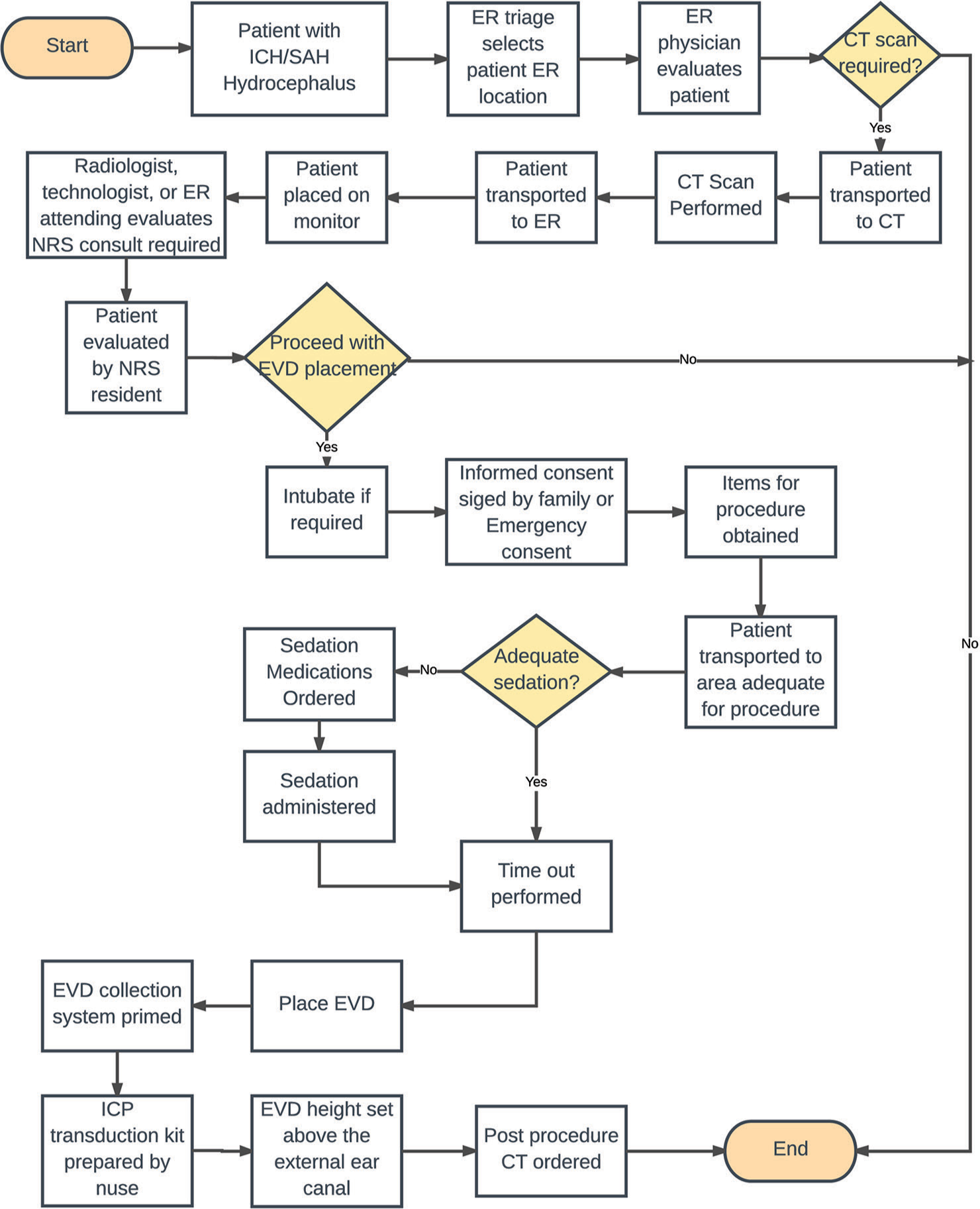
The conception of the EVD cart began with the collection of baseline data through surveys conducted at our institution between June 2017 and December 2017. Residents were asked to complete an anonymous survey regarding their comments on the EVD placement process and to record their individual time-to-EVD placement. The baseline self-estimated time-to-EVD data collected from residents were then corroborated with EMR time stamps. Additional surveys were conducted among ED nurses, ED attending physicians, CT staff, and trauma nurses on potential delays on time-toEVD. A root cause analysis framework was used to identify possible sources of delayed EVD placement. A process map as shown in [
EVD supply cart
A supply cart similar to a code cart was obtained and stocked with all necessary EVD supplies, including cranial access kit, burette collection system, ICP transduction kit, stapler, dressing kit, syringes, needles, and gauze [
Data collection and statistical analysis
Prospective data collection for patients undergoing EVD placement in the ED began in January 2018 after the development of the EVD cart. Retrospective review of patients who underwent EVD placement in the ED generated our control group. These patients were identified by reviewing resident case logs and neurological intensive care unit patient logs for patients who had an EVD placed in the ED; patients were excluded if the EVD was placed after arrival to the ICU or if the EVD was placed in another part of the hospital (e.g. in the trauma bay since ventriculostomy equipment is readily available there).
Time stamps as recorded in the EMR for the following events were collected: time of arrival and registration in the ED, time of initial head CT, time of neurosurgery consult (when recorded), and time of nursing procedure time out for EVD placement. Time-to-EVD was defined as the time from neurosurgical consultation to the time of the procedural time-out. In the case that time of neurosurgical consultation was not recorded, the time of initial head CT was used since neurosurgery is consulted immediately after head CT is performed in these patients. Procedure timeout was used as the endpoint to isolate the impact of the cart of preparation for EVD placement, irrespective of the time taken to place the EVD (which depends on the individual case and the practitioner placing the EVD). All EVDs were placed by neurosurgical resident physicians. To assess the impact of the intervention on clinical outcome, we also reviewed patient records to determine modified Rankin score (mRS) for each patient 1 year after EVD placement.
Median time to EVD was compared between the pre- and post-cart cohorts using one-way ANOVA and Kaplan–Meier analysis through the rank-sum test. Mean time to EVD was compared between groups using a two-tailed t-test. Difference in mean mRS score between the two groups was compared using a two-tailed t-test. Pre- and post-cart cohort patient demographics were compared using a two-sample t-test (age and arrival GCS), Fisher’s exact test (sex), and Chi-squared test (etiology). All statistical analyses comparing both cohorts were completed in MATLAB® (MathWorks®; Natick, MA, USA). Univariate linear regression and multiple linear regression were used to assess potential cohort demographic confounders on the time to EVD. Similarly, univariate logistic regression and multiple logistic regression were used to assess potential confounders on dichotomized mRS outcomes of each cohort. The patient ability to care for self was analyzed with the dichotomization of mRS with scores of 0–2 (able to care for self) versus scores of 3–6 (unable to care for self). The patient disability was assessed through dichotomization of mRS with scores of 0–1 (no significant disability) versus scores of 2–6 (any disability). Analysis for trends and special cause variations on case times to EVD were assessed through Statistical Process Control (SPC) charts. Regression and SPC chart analysis were completed in R (R Core Team, 2020). Power analysis was completed in G*Power (Faul, Erdfelder, Lang, and Buchner, 2007). P < 0.05 was set as a threshold for statistical significance.
RESULTS
The EVD supply cart was placed in the ED in January 2018. A total of 18 postcart patients who underwent EVD placement in the ED between January 2018 and October 2019 met our inclusion criteria and were included in our analysis. A total of 33 precart cases between April 2012 and January 2018 made up our control cohort. Thirteen patients were excluded from our postcart cohort and 71 patients were excluded from the control cohort based on our exclusion criteria. Time-to-EVD placement was compared between the control and the intervention groups. There was no significant difference between the two cohorts in age, there were significant differences in arrival GCS, sex, and etiology [
Patients underwent EVD placement for nontraumatic subarachnoid hemorrhage (aneurysmal in most cases) in 20 cases (10 in the precart and 10 in the postcart cohorts), nontraumatic intracerebral hemorrhage in 12 cases (6 in the precart and 6 in the postcart cohorts), and traumatic intracranial hemorrhage in 17 precart patients, isolated intraventricular hemorrhage in 1 postcart patient, and obstructive pineal mass in 1 postcart patient.
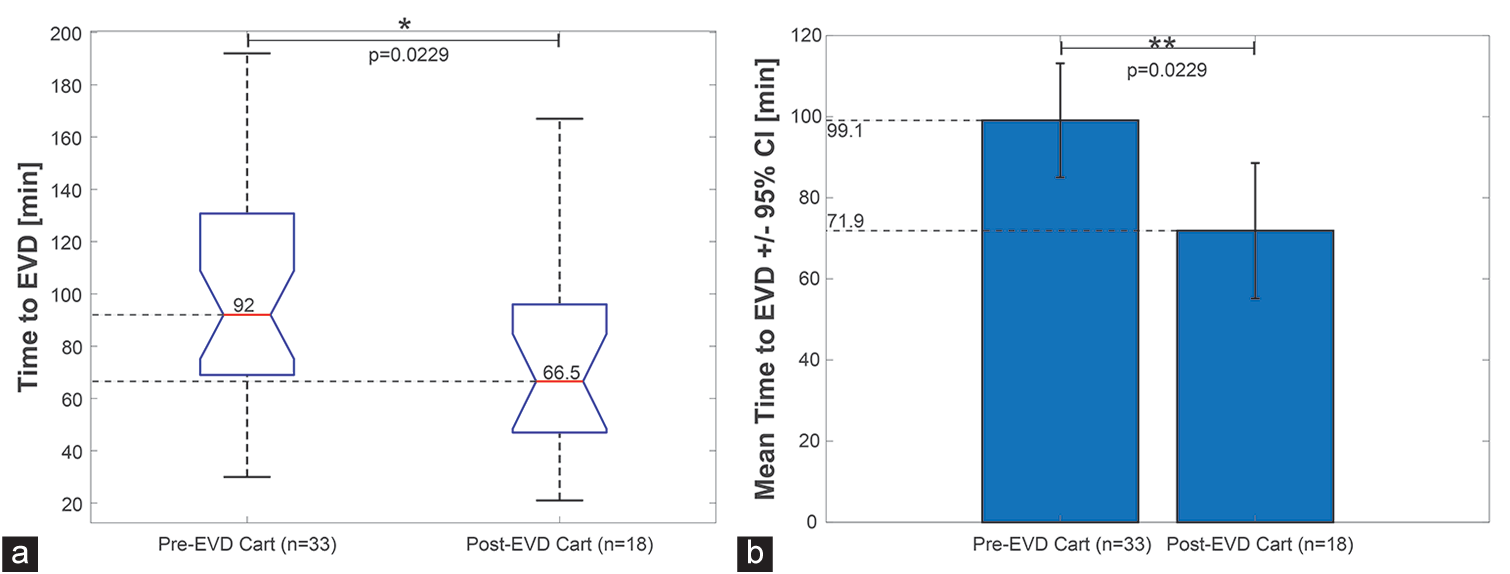
Time-to-EVD placement was decreased in our postcart cohort [
Figure 3:
Comparison of median and mean time-to-external ventricular drain (EVD) placement between the precart and postcart cohorts. (a) Boxplot showing the distribution of times to EVD placement with median and inner interquartile range (25–75% of the times) within the box outlined region. P-value corresponds to F-test. (b) Mean and 95% confidence interval on the mean for pre- and post-EVD cart groups. P-value corresponds to two-tailed t-test.
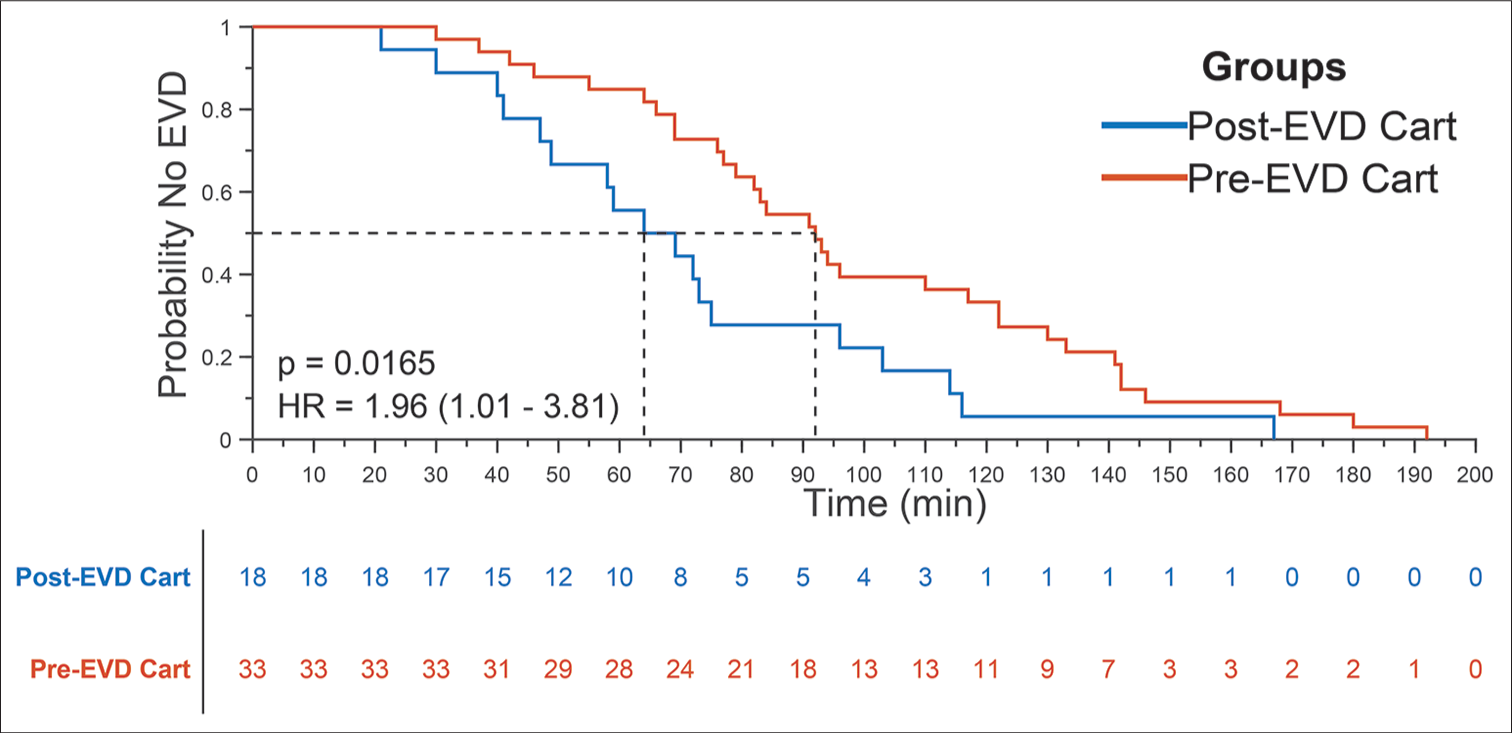
Figure 4:
Kaplan–Meier curve comparing time-to-external ventricular drain (EVD) placement before and after initiation of EVD supply cart. A Kaplan–Meier curve illustrating the probability of no EVD placement from time of patient presentation. The median time-to-EVD placement among the postcart cohort (n=18) was 64 min, representing a statistically significant (rank-sum test, P=0.0165) reduction in time from the median time of 92 min among the precart cohort (n=33). The median time was determined from the 0.5 probability of no EVD placement for both cohorts.
Individual case times to EVD were assessed with SPC charts. Twenty-five cases among the precart cohort had time-toEVD placement within one standard deviation of the mean, seven cases were within two standard deviations, and one case within three standard deviations [
We also sought to assess clinical impact of the intervention by comparing mRS score at 1 year after EVD placement between the pre- and post-cart groups. Postcart patients trended toward improved outcomes with lower mRS score at 1 year, but this did not reach statistical significance (two-tailed t-test, P = 0.177). Cohort demographic variations among arrival GCS and etiology did not have a statistically significant association with mRS scores (mRS 0–2 vs. mRS 3–6; patient able to care for self vs. not able to care for self) when analyzed through univariate logistic regression (P = 0.866 and P = 0.995–0.112, respectively). Cohort variations in sex did have a statistically significant relationship with mRS on univariate logistic regression (P = 0.042). When analyzing mRS regarding patient disability (mRS 0–1 vs. mRS 2–6; no significant disability vs. any disability), sex did not have a statistically significant association but did have a trend toward sex significance on univariate logistic regression (P = 0.0791). The multiple logistic regression on mRS outcomes at 1 year for cohort sex and the use of the EVD cart revealed no statistically significant results when analyzing for patient ability to care for self (P = 0.0895 and P = 0.5382, respectively; mRS 0–2 vs. mRS 3–6) or presence of disabilities (P = 0.126 and P = 0.755, respectively; mRS 0–1 vs. mRS 2–6). There were no unintended consequences as a result of the EVD cart. In addition, no patients or intervention data were lost to follow-up among the pre- and post-cart groups.
DISCUSSION
Root cause analysis for health-care quality improvement seeks to identify latent causes of avoidable adverse events or inefficiency.[
To the best of the authors’ knowledge, this is the first study of a dedicated EVD supply cart in the literature. Similar interventions have proven effective for other applications. Anesthesia medication carts have been shown to reduce the likelihood of medication-related adverse events, medication selection errors, and overall time spent searching for medication.[
Implementation of our EVD cart led to a statistically significant decrease in EVD placement time in the ED. The mean time-to-EVD placement in the postcart group was reduced by 27.21 min, representing a 27.46% reduction in overall time (P = 0.023), compared to the precart cohort. Median time-to-EVD placement was reduced by 28 min, 30.4% reduction (P = 0.017). The reduction in time-toEVD placement was not found to be related with variations in arrival GCS, sex, or etiology, but did have a statistically significant association with the use of the EVD cart. When analyzed through SPC charts, both cohorts exhibited behavior typical of stable processes, with no violation of Shewhart rules and no special cause variations. These results suggest that centralized supply plays a key role in directly reducing overall time to ventriculostomy.
We also observed a trend toward improved clinical outcomes in the postcart cohort based on mRS score at 1 year after EVD placement. However, these results did not reach statistical significance (P = 0.177). Differences in cohort sex did have a statistically significant association on the outcome of a patient’s ability to care for themselves (P = 0.042) and a trend toward significance on patient disability (P = 0.0791). In both cases, the use of the EVD cart, when accounting for the sex variable, did not have statistically significant associations with patient outcomes (P = 0.5382 and P = 0.755, respectively). This is potentially due to the wide range of pathologies in this study. A larger clinical cohort will be needed to ascertain the clinical impact of EVD placement times on complication rates and clinical outcome. Nonetheless, an EVD “crash cart” represents a simple and easy to implement intervention with the ability to significantly reduce time to ventriculostomy. There exists the potential that this intervention also improves overall clinical outcome, but more data will be needed to thoroughly assess this.
There are several limitations of this study, including the inherent limitation of a partially retrospective study and audit nature of the study design: our intervention group (postcart cohort) was compared to a control cohort that was drawn from several years of precart patients. All cases from both cohorts were subject to the same inclusion and exclusion criteria. In addition, the final sample size (n = 51) and achieved power of this study may impact the significance of the statistical comparisons conducted; based on the final observed effect size d of 0.7, a total of 66 cases (assuming equal allocation) would have been needed to obtain a statistical power of 0.8. Furthermore, many factors impact time-to-EVD placement (clinical decision-making, administration of blood products, speed of laboratory and pharmacy services, etc.), which can lead to substantial variability in time to ventriculostomy. Surgeons and ED staff were minimally aware that they were being timed and were not explicitly informed that time-to-EVD placement was being measured. That said, implementation of the EVD cart was a quality improvement initiative with the goal of improving time-to-EVD placement, thus it is possible that a Hawthorne effect impacted our findings. There exists the possibility that external factors contributed to improved times to EVD placement that is not directly related to the EVD cart (improved nursing or surgeon awareness, evolving practice patterns, and changes in resident comfort) and may confound our findings about impact of the EVD cart. Strict inclusion and exclusion criteria and large sample size help mitigate this concern.
As the EVD cart was designed for use in the ED, this study is limited by the fact that the influence of location of EVD placement on outcomes could not be assessed in this study. Increased risk of EVD-related infections has been proposed to be associated with EVD placement in the ED when compared to EVD placement in the operating room, however, there is no consensus in the literature.[
The generalizability of our results remains to be proven but represents an exciting opportunity for neurosurgeons to improve the care of some of their most acutely ill patients. Further, investigation and validation on data from other institutions are needed to thoroughly evaluate the generalizability of our intervention and better assess the effect on patient outcomes. Investigation of the clinical impact of the EVD cart would require a larger, more homogenous cohort. Expansion of this intervention to other institutions will allow us to test the generalizability of our results and better assess the effect on patient outcomes. This type of intervention may also be useful outside of neurosurgery, inspiring the creation of similar procedure supply carts for other applications throughout medicine.
CONCLUSION
Timely placement of an EVD is critical for the care of acute hydrocephalus.[






Dhaval Shukla
Posted September 30, 2022, 5:46 pm
not able to see supplementary figures, i am interested in developing the same at my institute
jim
Posted October 4, 2022, 10:00 am
Please check the PDF for the supplementary figures.
https://surgicalneurologyint.com/wp-content/uploads/2021/08/10976/SNI-12-362.pdf
Jim Cook
Managing Editor
Surgical Neurology International
31855 Date Palm Drive, Suite 3-400
Cathedral City, CA 92234
U.S.A.