- Department of Neurosurgery, Kesennuma City Hospital, Kesennuma, Japan.
- Department of Neurosurgery, Tohoku University Graduate School of Medicine, Sendai, Miyagi, Japan.
Correspondence Address:
Norio Narita
Department of Neurosurgery, Tohoku University Graduate School of Medicine, Sendai, Miyagi, Japan.
DOI:10.25259/SNI_887_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Masahito Katsuki1, Norio Narita1, Kanako Sato1, Ryuzaburo Kochi1, Taketo Nishizawa1, Kokoro Kawamura1, Naoya Ishida1, Ohmi Watanabe1, Siqi Cai1, Shinya Shimabukuro1, Teiji Tominaga2. Where to make burr hole for endoscopic hematoma removal against intracerebral hemorrhage at the basal ganglia to increase the hematoma removal rate – Comparison between trans-forehead and along-the-long-axis approaches. 03-Feb-2021;12:41
How to cite this URL: Masahito Katsuki1, Norio Narita1, Kanako Sato1, Ryuzaburo Kochi1, Taketo Nishizawa1, Kokoro Kawamura1, Naoya Ishida1, Ohmi Watanabe1, Siqi Cai1, Shinya Shimabukuro1, Teiji Tominaga2. Where to make burr hole for endoscopic hematoma removal against intracerebral hemorrhage at the basal ganglia to increase the hematoma removal rate – Comparison between trans-forehead and along-the-long-axis approaches. 03-Feb-2021;12:41. Available from: https://surgicalneurologyint.com/surgicalint-articles/10559/
Abstract
Background: Endoscopic hematoma removal is performed to treat intracerebral hemorrhage (ICH) at the basal ganglia. In our hospital, young neurosurgical trainees perform it for the only 1st to the 3rd time. We perform a “trans-forehead approach” and hypothesized that our technique would contribute to higher hematoma removal rate and easiness despite their inexperience. We compared our dataset with an open dataset with along-the-long-axis approaches using pre- and intraoperative neuronavigation by well-trained neurosurgeons and tested the utility of our trans-forehead approach.
Methods: We retrospectively investigated our 17 consecutive patients with hypertensive ICH who underwent endoscopic hematoma removal using the trans-forehead approach. We obtained the open dataset and compared our data with the 12 patients from the open dataset using the inverse probability weighting method. Operative time, hematoma removal rate, postoperative hematoma volume, Glasgow Coma Scale (GCS) on day 7, and modified Rankin Scale (mRS) at 6 months were assessed as outcomes.
Results: The median age was 68 (interquartile range; 58–78) years. Median postoperative hematoma volume, removal rate, operative time, GCS on day 7, and mRS at 6 months were 9 (2–24) mL, 90 (79–98)%, 53 (41–80) min, 13 (12–13), and 4 (2–5), respectively. The weighted generalized estimating equations revealed that operative time was shorter in the along-the-long-axis group, but other items were not significantly different between the two approaches.
Conclusion: The hematoma removal rate of endoscopic hematoma removal with the trans-forehead approach by young trainees was not different from that of the along-the-long-axis approach by well-trained neurosurgeons using neuronavigation.
Keywords: Endoscopic hematoma removal, Hematoma removal rate, Intracerebral hemorrhage, Less invasive surgery, Training of residents
INTRODUCTION
Intracerebral hemorrhages (ICHs) comprise 10–30% of all strokes and are strongly related to high mortality and morbidity.[
However, almost all of the patients underwent craniotomy in these previous studies; therefore, the benefit and efficacy of endoscopic hematoma removal remain unknown.
In 2016, Phase II of the Minimally Invasive Surgery with Thrombolysis for ICH Evacuation (MISTIE) trial showed favorable preliminary results for the stereotactic aspiration and catheter drainage with a tissue plasminogen activator.[
Considering these previous studies on minimally invasive surgery, although the effect of endoscopic surgery for intracerebral hematoma remains unclear, a higher removal rate of hematoma would be important for outcome improvement.[
In our hospital, young neurosurgical trainees of 3–7 years perform endoscopic hematoma removal under a mentor’s supervision over 60 years old, who were not skill qualified by the Japanese Society for Neuroendoscopy.[
MATERIALS AND METHODS
Study population
From the medical records between 2013 and 2020, we retrospectively investigated 17 consecutive patients with hypertensive ICH who underwent endoscopic hematoma removal. The ICH diagnosis was based on the clinical history and the presence of ICH on computed tomography (CT). The inclusion criteria for the study were as follows: (1) patients with ICH at the basal ganglia, (2) patients indicated for surgical treatment according to the Japanese Guidelines for the Management of Stroke 2009[
General management
All patients received standard management according to the Japanese Guidelines for the Management of Stroke 2009[
Neuroendoscopic procedure
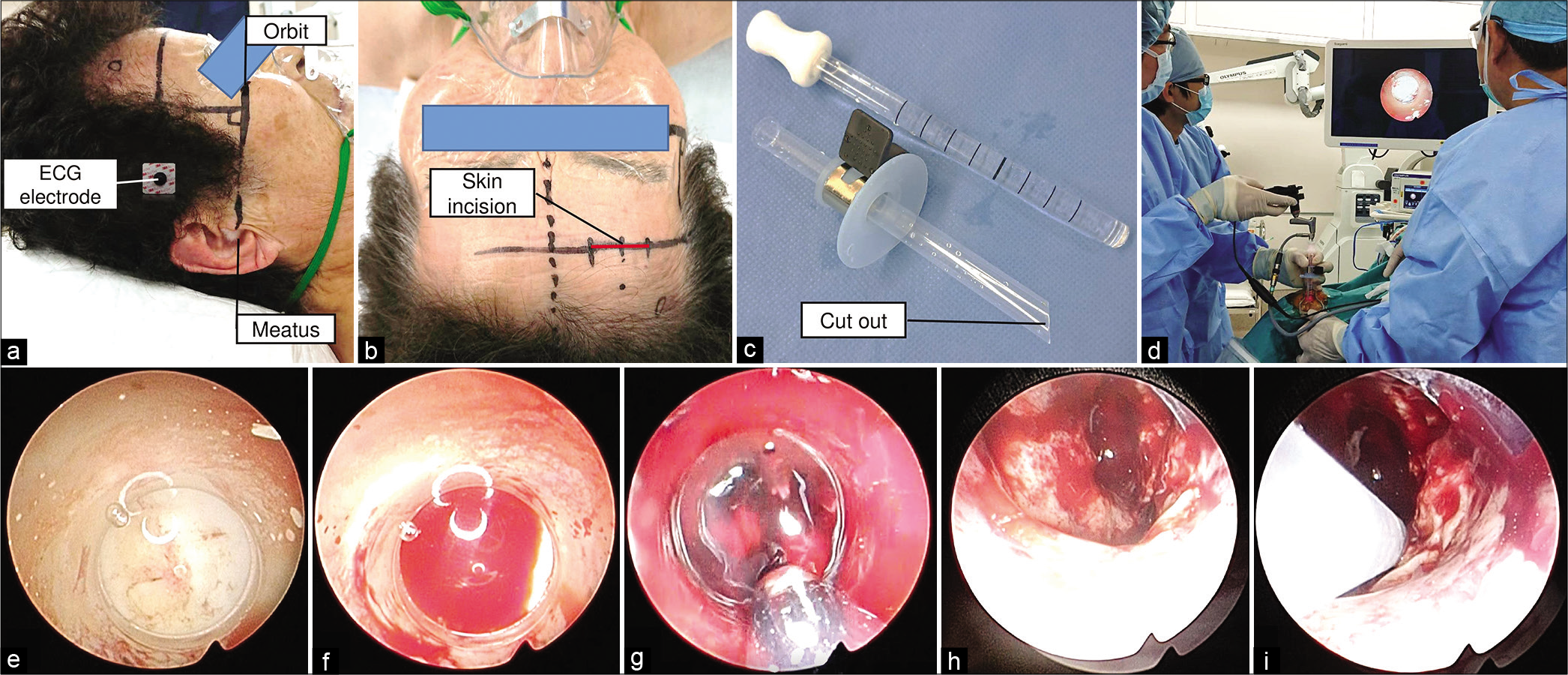
We have performed trans-forehead endoscopic procedures regardless of age, comorbidities, and presence of antithrombotic drugs. Endoscopic hematoma removal was performed under local anesthesia. We also prepared for conversion to craniotomy under general anesthesia simultaneously just in case the brain expanded rapidly or hemostasis was difficult endoscopically. The patient’s head was placed on the horseshoe headrest. We first confirmed the orbitomeatal line (OM line). We then checked the CT images slice in which the hematoma was described most vigorously and write its line parallel to the OM line. As the mark, the electrocardiogram electrode was fixed [
Figure 1:
Intraoperative findings. We first confirmed the orbitomeatal line (OM line). We then checked the computed tomography images slice in which the hematoma was described most vigorously and write its line parallel to the OM line. As the mark, the electrocardiogram electrode was fixed (a). A 3 cm skin incision along the wrinkling and burr hole 3–4 cm outside the midline were made parallel to the cross-sectional line of the CT slice (b). After a cruciate dural incision and corticotomy, we prepared a transparent sheath. The stopper was clamped so that the sheath’s tip reached 1/3 of the length from the hematoma’s deepest part. We made the cut out and the clamp orientation the same direction as a mark (c). A neurosurgeon introduced the sheath with the observation by the rigid endoscope through the sheath. The ECG electrode helps to determine the inserting orientation, and we just considered the lateral angle (d). First, we saw the white matter (e), and then, we saw the red hematoma cavity and confirmed the reach into the hematoma (f). The sheath was inserted to the stopper’s preclamped position, and we removed the hematoma by the suction cannula. We did not aggressively change the sheath’s direction but just rotated the sheath and removed the hematoma that came out naturally into the sheath (g). We refrained from aggressive hematoma removal near the internal capsule to save the pyramidal tract, which was not destroyed by hemorrhage and left some part of the hematoma (h). After hematoma removal, we reinsert the sheath and left the drainage tube (i). The burr hole was covered with the burr hole cover, and the skin was sutured.
Clinical variables and outcomes
We collected data regarding physiological symptoms and medical history on admission; age, sex, hematoma location, Glasgow Coma Scale (GCS) score, systolic blood pressure on admission, presence of smoking, heavy drinking, comorbidities, use of antithrombotic drugs, and operative time. We also measured the hematoma volume and the hematoma removal rate from the head CT on admission and just after the operation. The hematoma volume was calculated by the ABC/2 method.[
Comparative data
We gained the comparative data from the open dataset of Suwa Red Cross Hospital.[
Statistical analysis
Results were presented as median (interquartile range). We investigated all the 29 ICH patients focusing on the clinical variables described above, using the Mann–Whitney U-test and Fisher’s exact test as univariate analysis. We performed the inverse probability weighting (IPW) method to address confounding by observed covariates, a type of propensity score analysis. The propensity score was calculated by a logistic regression model predicting treatment (trans-forehead or along-the-long-axis approaches) and adjusting the baseline characteristics of age, sex, past history, systolic blood pressure on admission, GCS score on admission, and preoperative hematoma volume as independent variables. The calculated probability of receiving the treatment was evaluated by the Hosmer–Lemeshow test and c-statistic. The weights for each patient were calculated as the inverse of the probability of receiving the treatment. After balancing with the IPW method, we made generalized estimating equations to assess the association between the surgical procedure and each outcome. We conducted these analyses using version 21.0.0 of SPSS software (IBM, NY, USA). A two-tailed P < 0.05 was considered as statistically significant.
RESULTS
General characteristics
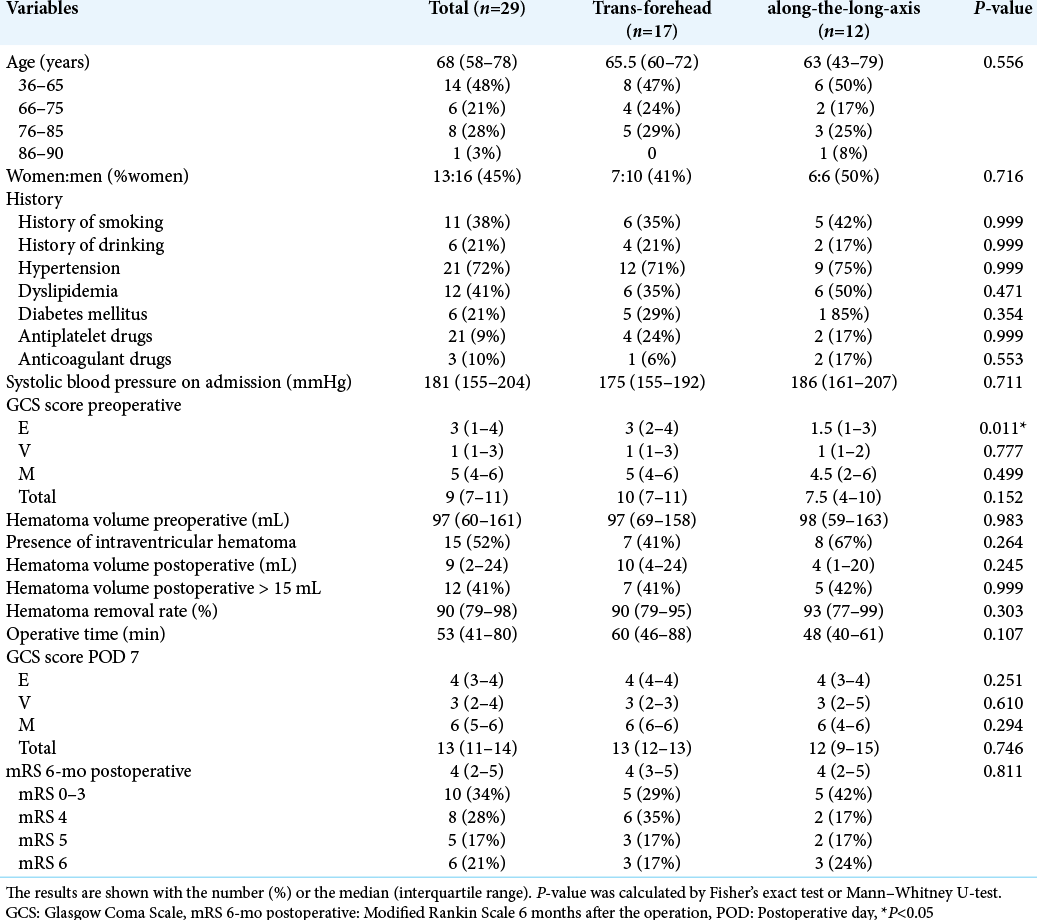
[Tbale 1] shows the characteristics of 29 patients (17 trans-forehead approach and 12 along-the-long-axis approach) with ICH at the basal ganglia. Thirteen women and 16 men were included. The median age was 68 (58–78) years. Median postoperative hematoma volume, removal rate, operative time, GCS on day 7, and mRS at 6 months were 9 (2–24) mL, 90 (79–98)%, and 53 (41–80) min, 13 (12-13), and 4 (2–5), respectively. Only the preoperative GCS E score was significantly worse in the along-the-long-axis group (P = 0.011). Other variables were not significantly different between the two datasets. In all 29 patients, no one had chronic renal failure treated with hemodialysis or liver cirrhosis, reported as related to the hematoma removal rate.[
Among both patients who underwent trans-forehead approach and along-the-long-axis approach, no one needed conversion to craniotomy during endoscopic hematoma removal. All the patients could undergo endoscopic surgery under local anesthesia without the patient’s body movement, change of the vital signs, or worsening respiratory conditions.
Comparison of the trans-forehead approach and along-the-long-axis approach using IPW method
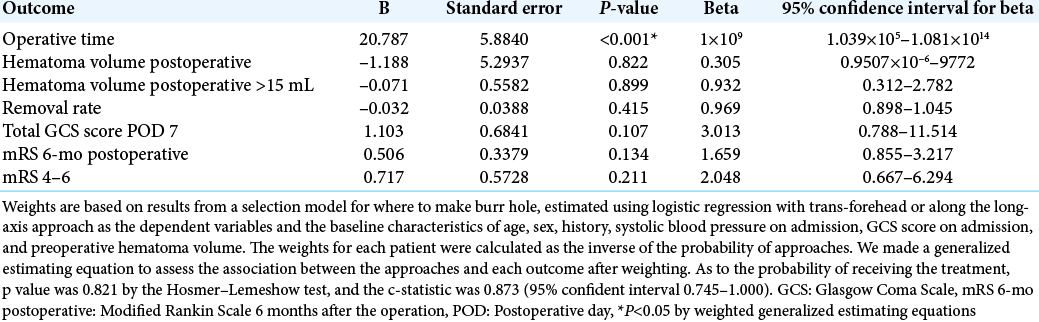
Regarding the probability of receiving the treatment, P-value was 0.821 according to the Hosmer–Lemeshow test, and the c-statistic was 0.873 (95% confident interval 0.745–1.000). The weighted generalized estimating equations revealed that operative time was shorter in the along-the-long-axis group (P < 0.001). However, postoperative hematoma volume, removal rate, GCS score on day 7, and mRS at 6 months were not significantly different between the two approaches [
Representative case
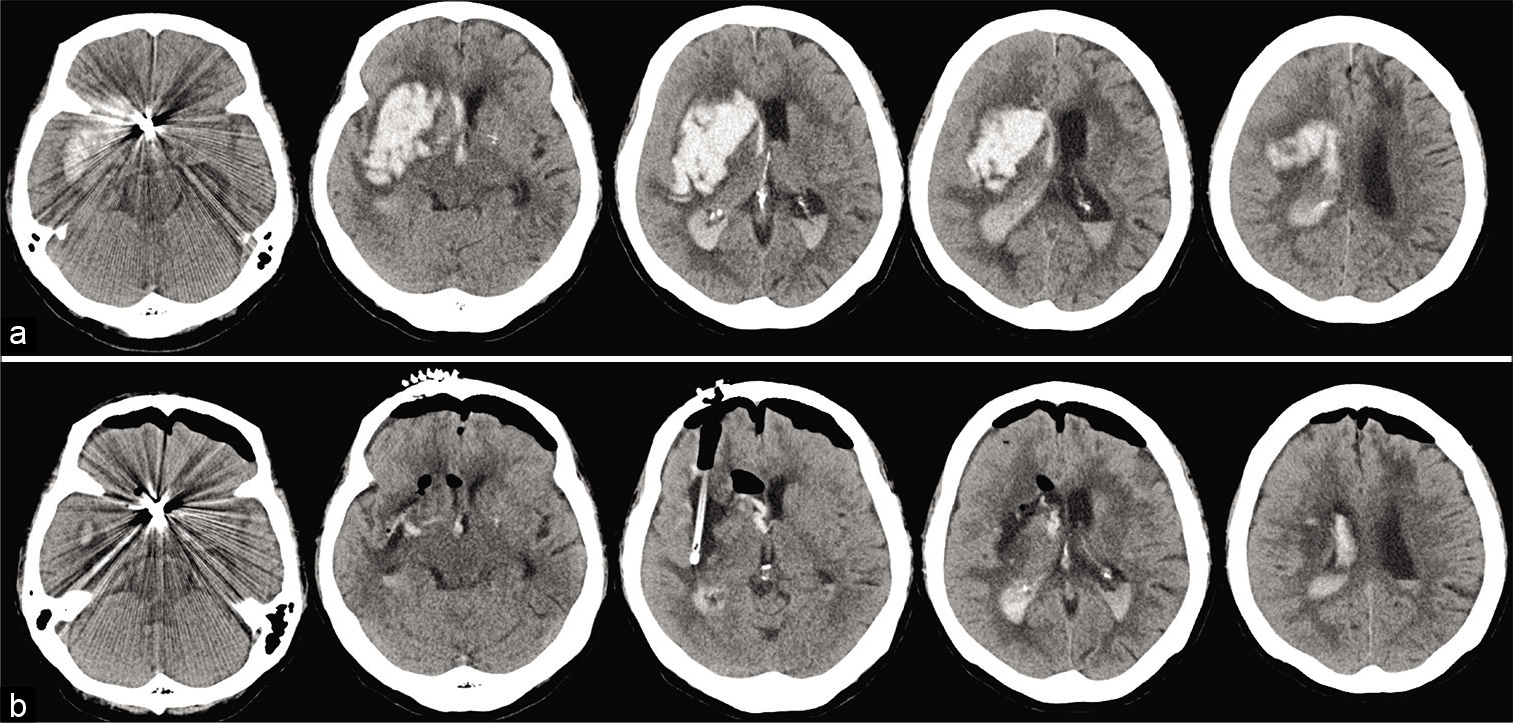
A 79 woman presented with the left hemiplegia and her GCS score was 7 (E2V1M4) on admission. CT showed 75 mL of the right putaminal hemorrhage [
Figure 2:
Representative case: a 79 woman presented with the left hemiplegia, and her GCS score was 7 (E2V1M4) on admission. The preoperative CT showed 75 mL of the right putaminal hemorrhage (a). Endoscopic hematoma removal with a trans-forehead approach was performed. Postoperative CT showed 3 mL of the rest, and the hematoma removal rate was 96% (b).
DISCUSSION
The concept of our surgical strategy is as follows: (1) easiness for young trainees by inserting sheath parallel to the CT slice to understand the orientation, (2) conserve white matter by refraining from aggressively changing the sheath’s direction but just rotating the sheath and removed the hematoma that came out naturally into the sheath, and (3) not requiring specialized tools for endoscopic hematoma removal,[
Where to make burr hole
Burr hole for endoscopic hematoma removal at the basal ganglia is often made at the frontal region for trans-forehead or along-the-long-axis (frontal approaches), near the Kocher’s point, or on the point which was shortest to the hematoma (temporal approach). Hsieh et al. investigated the efficacy of the two entry sites (frontal or temporal approach), and they concluded that the frontal approach could facilitate the optimal evacuation of putaminal hemorrhage. The frontal approach is through the noneloquent area, and the hematoma evacuation rate can be better than the temporal approach because the visualization of the frontal part of the hematoma might be blocked due to the limited inclination of the tube through a small burr hole in the temporal approach.[
Yokosuka et al. reported a freehand technique with making a burr hole near the Kocher’s point, which is used for the cerebral ventricular drainage[
Disadvantages of our technique
Sometimes, we could not take the sheath into the hematoma by one trial and injured the white matter. Neuronavigation enables us to precisely reach into the hematoma by one trial and avoid injuring the eloquent area and vessels while making the entry tract.[
Limitation
Our study’s sample size was small, and quantification of the hematoma volume by ABC/2 methods is not so exact. Thus, a prospective, multicenter study with a large number of patients is needed to evaluate the difference of where to make a burr hole. However, every surgical procedure has its advantages and disadvantages. It is impossible to say which method is superior for endoscopic hematoma removal. We hope that the trans-forehead approach would help us when there is no navigation or when a young neurosurgeon is unfamiliar with the endoscopic hematoma removal for ICH at basal ganglia.
CONCLUSION
The hematoma removal rate of endoscopic hematoma removal with the trans-forehead approach for ICH at the basal ganglia by young trainees was not different from that of the along-the-long-axis approach well-trained neurosurgeons using neuronavigation.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Bernardo F, Rebordão L, Machado S, Salgado V, Pinto AN. In-hospital and long-term prognosis after spontaneous intracerebral hemorrhage among young adults aged 18-65 years. J Stroke Cerebrovasc Dis. 2019. 28: 104350
2. Gebel JM, Sila CA, Sloan MA, Granger CB, Weisenberger JP, Green CL. Comparison of the ABC/2 estimation technique to computer-assisted volumetric analysis of intraparenchymal and subdural hematomas complicating the GUSTO-1 trial. Stroke. 1998. 29: 1799-801
3. Hanley DF, Thompson RE, Muschelli J, Rosenblum M, McBee N, Lane K. Safety and efficacy of minimally invasive surgery plus alteplase in intracerebral haemorrhage evacuation (MISTIE): A randomised, controlled, open-label, phase 2 trial. Lancet Neurol. 2016. 15: 1228-37
4. Hanley DF, Thompson RE, Rosenblum M, Yenokyan G, Lane K, McBee N. Efficacy and safety of minimally invasive surgery with thrombolysis in intracerebral haemorrhage evacuation (MISTIE III): A randomised, controlled, open-label, blinded endpoint phase 3 trial. Lancet. 2019. 393: 1021-32
5. Hayashi T, Karibe H, Akamatsu Y, Narisawa A, Shoji T, Sasaki T. Endoscopic hematoma evacuation for intracerebral hemorrhage under local anesthesia: Factors that affect the hematoma removal rate. World Neurosurg. 2019. 126: e1330-6
6. Hemphill JC, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M. Guidelines for the management of spontaneous intracerebral hemorrhage: A guideline for healthcare professionals from the American Heart Association/ American Stroke Association. Stroke. 2015. 46: 2032-60
7. Hsieh PC, Cho DY, Lee WY, Chen JT. Endoscopic evacuation of putaminal hemorrhage: How to improve the efficiency of hematoma evacuation. Surg Neurol. 2005. 64: 147-53
8. Skill Qualified Doctors by Japanese Societiy for Neuroendoscopy. Available from: http://www.square.umin.ac.jp/jsne/qualifications.html [Last accessed on 2020 Sep 04].
9. Katsuki M, Kakizawa Y, Nishikawa A, Yamamoto Y, Uchiyama T. Lower total protein and absence of neuronavigation are novel poor prognostic factors of endoscopic hematoma removal for intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2020. 29: 105050
10. Katsuki M, Kakizawa Y, Nishikawa A, Yamamoto Y, Uchiyama T. The dataset on the characteristics of the intracerebral hemorrhage patients treated by endoscopic hematoma removal or craniotomy. Data Br. 2020. 33: 106387
11. Katsuki M, Kakizawa Y, Nishikawa A, Yasunaga Y, Uchiyama T. Endoscopic hematoma removal of supratentorial intracerebral hemorrhage under local anesthesia reduces operative time compared to craniotomy. Sci Rep. 2020. 10: 10389
12. Keep RF, Hua Y, Xi G. Intracerebral haemorrhage: Mechanisms of injury and therapeutic targets. Lancet Neurol. 2012. 11: 720-31
13. Kellner CP, Song R, Pan J, Nistal D, Scaggiante J, Chartrain AG. Long-term functional outcome following minimally invasive endoscopic intracerebral hemorrhage evacuation. J Neuro Intervent Surg. 2020. 12: 489-94
14. Mendelow AD, Gregson BA, Fernandes HM, Murray GD, Teasdale GM, Hope DT. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the international surgical trial in intracerebral haemorrhage (STICH): A randomised trial. Lancet. 2005. 365: 387-97
15. Mendelow AD, Gregson BA, Rowan EN, Murray GD, Gholkar A, Mitchell PM. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): A randomised trial. Lancet. 2013. 382: 397-408
16. Mould WA, Carhuapoma JR, Muschelli J, Lane K, Morgan TC, McBee NA. Minimally invasive surgery plus recombinant tissuetype plasminogen activator for intracerebral hemorrhage evacuation decreases perihematomal edema. Stroke. 2013. 44: 627-34
17. Nagasaka T, Tsugeno M, Ikeda H, Okamoto T, Inao S, Wakabayashi T. Early recovery and better evacuation rate in neuroendoscopic surgery for spontaneous intracerebral hemorrhage using a multifunctional cannula: Preliminary study in comparison with craniotomy. J Stroke Cerebrovasc Dis. 2011. 20: 208-13
18. Nishihara T, Nagata K, Tanaka S, Suzuki Y, Izumi M, Mochizuki Y. Newly developed endoscopic instruments for the removal of intracerebral hematoma. Neurocrit Care. 2005. 2: 67-74
19. Sacco S, Marini C, Toni D, Olivieri L, Carolei A. Incidence and 10-year survival of intracerebral hemorrhage in a population-based registry. Stroke. 2009. 40: 394-9
20. Safatli DA, Günther A, Schlattmann P, Schwarz F, Kalff R, Ewald C. Predictors of 30-day mortality in patients with spontaneous primary intracerebral hemorrhage. Surg Neurol Int. 2016. 7: S510-7
21. .editors. Japanese Guidelines for the Management of Stroke 2009. Tokyo: Kyowa Kikaku; 2009. p.
22. .editors. Japanese Guidelines for the Management of Stroke 2015. Tokyo: Kyowa Kikaku; 2015. p.
23. Vespa P, Hanley D, Betz J, Hoffer A, Engh J, Carter R. ICES (Intraoperative Stereotactic Computed Tomography-Guided Endoscopic Surgery) for brain hemorrhage: A multicenter randomized controlled trial. Stroke. 2016. 47: 2749-55
24. Wang Q, Guo W, Liu Y, Shao W, Li M, Li Z. Application of a 3D-printed navigation mold in puncture drainage for brainstem hemorrhage. J Surg Res. 2020. 245: 99-106
25. Wu R, Qin H, Cai Z, Shi J, Cao J, Mao Y. The clinical efficacy of electromagnetic navigation-guided hematoma puncture drainage in patients with hypertensive basal ganglia hemorrhage. World Neurosurg. 2018. 118: e115-22
26. Yokosuka K, Uno M, Hirano K, Toi H, Matsuzaki K, Matsubara S. Freehand technique for putaminal hemorrhage. Neurol Med Chir (Tokyo). 2011. 51: 543-6