- Departments of Radiology, Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil
- Departments of Neuroradiology, Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil
- Departments of Neuropathology, Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil
- Department of Neurosurgery, University Hospital Sao Francisco de Paula, Pelotas, Brazil
- Departments of Neurosurgery, Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil
- Departments of Neurology, Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil
Correspondence Address:
Matheus D. Soldatelli
Departments of Radiology, Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil
Departments of Neuroradiology, Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil
DOI:10.25259/SNI_179_2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Matheus D. Soldatelli, Francine Hehn de Oliveira, Amália Izaura Nair de Medeiros Klaes, Rafael Sodré da Silva, Ápio Cláudio Martins Antunes, Marino Muxfeldt Bianchin, Juliana Ávila Duarte. Xanthogranulomatous colloid cyst: Radiologic– pathologic correlation and diagnostic difficulties. 30-Aug-2019;10:169
How to cite this URL: Matheus D. Soldatelli, Francine Hehn de Oliveira, Amália Izaura Nair de Medeiros Klaes, Rafael Sodré da Silva, Ápio Cláudio Martins Antunes, Marino Muxfeldt Bianchin, Juliana Ávila Duarte. Xanthogranulomatous colloid cyst: Radiologic– pathologic correlation and diagnostic difficulties. 30-Aug-2019;10:169. Available from: http://surgicalneurologyint.com/surgicalint-articles/9600/
Abstract
Background: Despite colloid cyst in the third ventricle is a very usual cause of hydrocephalus, its xanthogranulomatous variant is rare. The most important differential diagnosis is the third ventricular craniopharyngioma. To the best of the authors’ knowledge, there have been few cases of xanthogranulomatous variant colloid cysts reported in the English literature.
Case Description: A 77-year-old white woman presented with headaches, memory loss, and abnormal gait for the past 4 months. Magnetic resonance imaging revealed a solid cystic lesion measuring 3.0 cm×2.8 cm×2.9 cm located inside the anterior portion of the third ventricle causing obstructive hydrocephalus. The posterior portion of the lesion was predominantly solid and hypointense on T2 and T1, with areas of post- contrast enhancement, and the anterior portion was predominantly cystic with both hyper- and hypointense areas on T1 and T2, with no suppression on fluid-attenuated inversion recovery and no restriction to diffusion. The patient underwent a left frontal craniotomy with pterional approach, and the lesion was removed microsurgically.
Conclusion: Xanthogranulomatous reaction is rarely described in colloid cysts, which happens as a response to desquamation of epithelial lining, subsequent lipid accumulation, and as tissue inflammatory response to intracystic hemorrhage. Microsurgical resection is the treatment of choice. As compared to the plain colloid cyst, these lesions are difficult to fully excise as the inflammatory reaction to the xanthomatous material leads to adhesions to adjacent structures; therefore, the aspiration of cystic contents without spillage is advisable to achieve maximal resection of cyst walls.
Keywords: Mimics, Neuroimaging, Neuroradiology, Neurosurgery, Pathology
INTRODUCTION
Although colloid cysts are the most common benign neoplasm of the anterior third ventricle, its xanthogranulomatous transformation has scarcely been reported in the literature and may impact adequate patient management. Here, we report a rare case of atypical colloid cyst treated with neurosurgery and review the most significant imaging and surgical findings.
CASE REPORT
History
A 77-year-old white woman presented to the emergency room with complaints of headaches, memory loss, and abnormal gait for the past 4 months. She had a 10-year history of a controlled type 2 diabetes mellitus and hypertension. At neurological examination, she was awake, partially oriented, with pupils equal round and reactive to light, without papilledema, perceived strength, eumetric intention tremor, no frontal release signs, and ataxia of gait.
Radiographic features
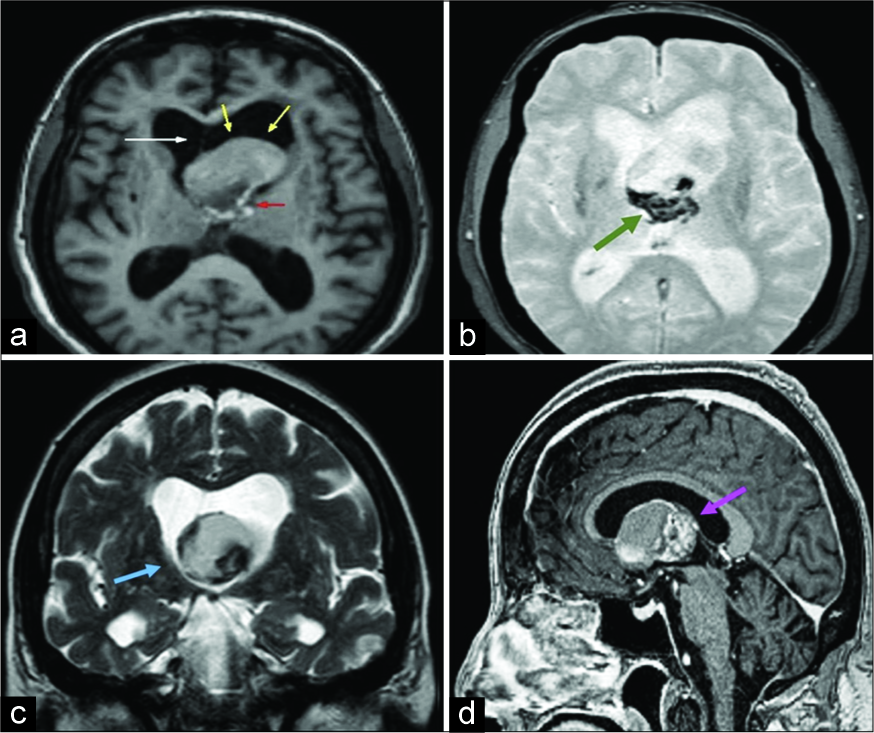
A magnetic resonance imaging (MRI) revealed a hyperintense T1WI solid cystic lesion measuring 3.0 cm×2.8 cm×2.9 cm located inside the anterior portion of the third ventricle causing obstructive hydrocephalus. The posterior portion of the lesion was predominantly solid and hypointense on T2WI and on gradient echo (GRE) T2WI, and the anterior portion was predominantly cystic with both hyper- and hypointense areas on T1WI and T2WI, with no suppression on fluid- attenuated inversion recovery (FLAIR) and no restriction to diffusion. The solid portion was hypointense on T1WI and demonstrated heterogeneous areas of enhancement after gadolinium contrast agent administration; there were also some peripheral sparse areas of enhancement in the cystic portion of the lesion. These heterogeneous peripheral enhancements and hypointensity on GRE TW2I are consistent with either hemosiderin or calcifications, raising concern for an atypical colloid cyst or a cystic craniopharyngioma [
Figure 1:
Axial T1 (a) and T2 gradient echo (b); coronal T2WI (c) and sagittal post-Gadolinium T1W1 (d). Heterogeneous solid- cystic mass obstructing the third ventricle, located at the foramen of Monro (blue arrow), at the anterosuperior aspect of the third ventricle, causing a deviation of the midline (white arrow) and obstructive hydrocephalus. The anterior portion of the lesion is predominantly cystic with a moderate hyperintense signal (yellow arrows). The posterior portion is predominantly solid (red arrow), has hypointense areas suggestive of either calcifications or a hemorrhagic component (green arrow), and shows heterogeneous enhancement (pink arrow).
Surgical management
A left frontal craniotomy with pterional approach and a transfrontal route using endoscopic visualization and stereotactic guidance were performed, and the intraventricular lesion was removed microsurgically. The solid part of the lesion was excised, and most of the capsules were removed except for the part densely adherent to the floor of the third ventricle. About 3–4 ml of “machine-like oil” fluid was also drained.
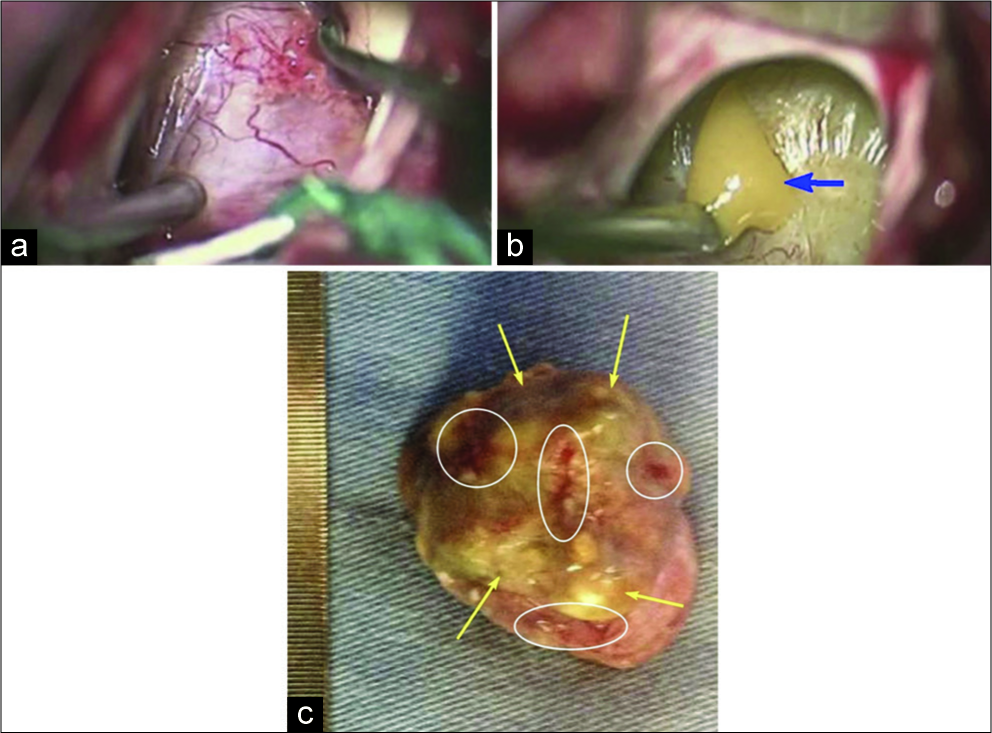
In the intraoperative view, the lesion was solid cystic located in the third ventricle near the foramen of Monro. The specimen received and fixed in 10% buffered formalin, displayed a nodular appearance, measured 2.5 cm×2.0 cm×2.0 cm, and weighed 7.0 g. It was surrounded by a delicate irregular membrane, with a yellowish color and friable areas. The solid part was firm, grayish-black in color, and avascular [
Figure 2:
Intraoperative images (a and b) microsurgical removal of the colloid cyst’s capsule (blue arrow) using endoscopic visualization and resection of the colloid cyst (b). Gross specimen (c) displaying nodular appearance surrounded by a delicate irregular membrane, with a yellowish color (yellow arrows) and friable areas (inside white ellipses), size: 2.5 cm×2.0×2.0cm and weigh: 7.0 g.
Pathology
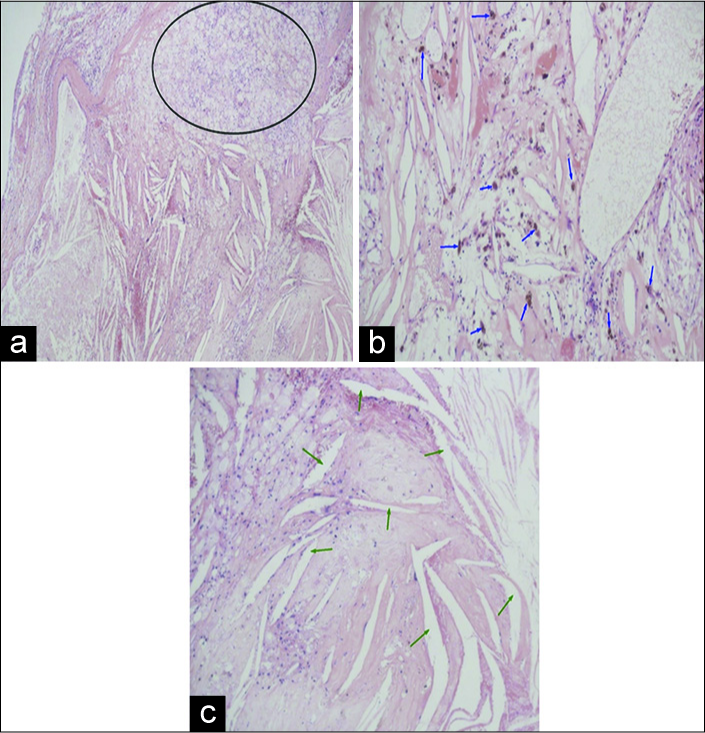
In the intraoperative histological examination, a diagnosis of craniopharyngioma was made, but the definitive histopathological examination of the excised specimen revealed a colloid cyst lined by a single layer of cuboidal, partially flattened nonciliated and ciliated epithelial cells. There was a high prevalence of macrophages and epithelioid cells, many of which included refractile material and hemosiderin. In addition, cholesterol clefts and lymphocytes were seen in focal sets. Polymorphonuclear leukocytes were diffusely spread out throughout the granuloma [
Figure 3:
Photomicrograph (hematoxylin-eosin stain, magnification ×4.0 [a] and ×20.0 [b and c]) images of the specimen: colloid cyst lined by a single layer of cuboidal, partially flattened non- ciliated and ciliated epithelial cells. There was a high prevalence of macrophages and epithelioid cells (inside black ellipse), many of which included refractile material and hemosiderin (blue arrows); polymorphonuclear leukocytes were diffusely spread out throughout the granuloma. In addition, cholesterol clefts (green arrows) and lymphocytes were seen in focal sets.
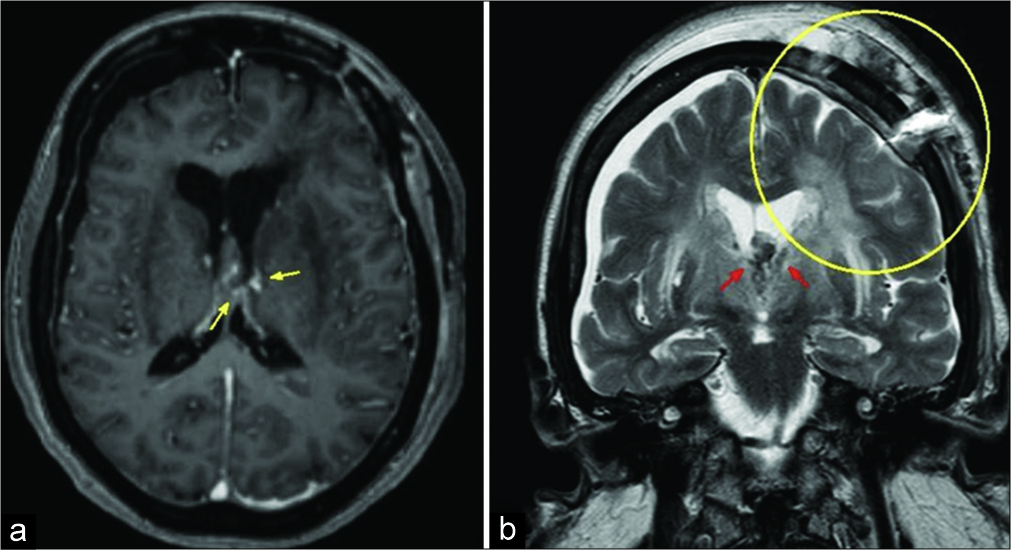
Figure 4:
The immediate post-operative axial T1WI (a) demonstrated an almost complete reduction of the lesion, with some sparse areas of contrast agent enhancement (yellow arrows). There is normalization in the lateral ventricle size. Coronal T2WI (b) shows post-surgical changes in the left frontal region (inside the yellow circle). There is hypointense material inside the ventricular system consistent with the degradation of hemoglobin (red arrows).
DISCUSSION
Colloid cysts account for 0.5%–1% of all intracranial tumors, with a yearly incidence of 3.2 cases per 1 million people; however, they are the most common benign neoplasm of the anterior third ventricle. The peak incidence is around the forties, and there is no gender prevalence[
The congenital nature of colloid cyst is usually accepted, with origins ranging from primitive neurectoderm, choroid plexus, and ependyma. At gross pathology, the colloid cysts are usually ovoid well-delineated cysts with a thick gelatinous center with variable viscosity with rare evidence of hemorrhage. At microscopy, these tumors are usually described as being cystic and lined by a single or multilayered ciliated, cuboidal epithelium. A xanthogranulomatous reaction is rarely described in these cysts, which happens as a response to desquamation of epithelial lining and subsequent lipid accumulation as well as tissue inflammatory response to intracystic hemorrhage. Xanthogranulomas are seen as cholesterol crystal clefts, foreign-body giant cells, foamy cells, and inflammatory infiltrate of varying degrees located in the wall of colloid cysts.[
Computed tomography (CT) studies showed that colloid cysts are hyperdense in two-thirds of cases and iso- to hypodense in one-third of cases, with density depending on the cholesterol content of the lesion. It usually does not enhance on contrast except for occasional rim enhancement.
On MRI, the signal varies according to the contents of the cyst, the characteristic appearance of being hyperintense on T1W images reflects the amount of cholesterol content, and the iso- to hypointensity on the T2W images is given by the amount of dense or proteinaceous fluid. There may be a peripheral (rim) gadolinium enhancement in a minority of cases. These colloid cysts do not normally suppress on FLAIR studies and show no restriction on diffusion-weighted imaging.
Both CT and MRI studies may not be able to distinguish between colloid cysts and its xanthogranulomatous transformation; however, atypical features should alert for this differential diagnosis. The presence of blood within the cyst can trigger to xanthogranulomatous inflammatory response, which is seen as heterogeneous signal intensity on MRI; in these cases, signal characteristics are variable and depend on the mixture of lipid, fluid, and blood products.[
The presence of an atypical solid-cystic lesion in the third ventricle near the foramen of Monro, as in our case, can mimic a suprasellar cystic craniopharyngioma, especially the papillary subtype (considering patient’s age), although such neoplasms are rarely presented in the third ventricle and more often have calcifications and gadolinium enhancement. Xanthogranulomas are often present in the atria of lateral ventricles and rarely can manifest in the third ventricle. They should be taken into consideration in the differential diagnosis of xanthogranulomatous colloid cysts, with iso- to a hyperintense signal on T1W images and variable gadolinium enhancement patterns. However, they have similar features and may be indistinguishable on imaging. In addition, xanthogranulomatous changes within colloid cysts or mixed tumors consisting of both of colloid cysts and xanthogranuloma have been described.
Other lesions, such as subependymoma and choroid plexus papilloma, can also be located in the foramen of Monro; however, the first is usually located at the 4th ventricle and demonstrates T2/FLAIR hyperintensity, and the second typically manifests in early childhood with an enhancing lobulated (cauliflower-like) mass in the atrium of the lateral ventricle.
Surgery is the treatment of choice, which can be accomplished either by endoscopic or microscopic techniques according to the expertise of the surgeon. As compared to the plain colloid cyst, these lesions are difficult to fully excise as the inflammatory reaction to the xanthomatous material leads to adhesions to adjacent structures, such as the walls of the ventricles.[
The prognosis for xanthogranulomatous colloid cyst is usually good, depending on the amount of resected tissue. The most common complications, such as aseptic meningitis and vasospasm, arise post-operatively and are due to spillage of the cyst contents.[
CONCLUSION
We reported a rare case of xanthogranulomatous colloid cyst of the third ventricle. Although computed tomography and magnetic resonance imaging may not be able to provide high specificity for this diagnosis, the presence of atypical neuroimaging features should raise concern, since this entity impose neurosurgery difficulties and its prognosis depend on the amount of resected tissue.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understands that her name and initials will not be published, and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to show our gratitude to the Neurosurgery and Radiology staff for collaborating in providing great insights in this case report.
References
1. Alugolu R, Chandrasekhar YB, Shukla D, Sahu BP, Srinivas BH. Xanthogranulomatous colloid cyst of the third ventricle. J Neurosci Rural Pract. 2013. 4: 183-6
2. Antunes JL, Kvam K, Ganti SR, Louis KM, Goodman J. Mixed colloid cysts-xanthogranulomas of the third ventricle. Surg Neurol. 1981. 16: 256-61
3. Carrasco R, Pascual JM, Medina-López D, Burdaspal-Moratilla A. Acute hemorrhage in a colloid cyst of the third ventricle: A rare cause of sudden deterioration. Surg Neurol Int. 2012. 3: 24-
4. Hadfield MG, Ghatak NR, Wanger GP. Xanthogranulomatous colloid cyst of the third ventricle. Acta Neuropathol. 1985. 66: 343-6
5. Kudesia S, Das S, Shankar SK, Santosh V, Reddy AK. Colloid cyst xanthogranuloma of the third ventricle a case report. Indian J Pathol Microbiol. 1996. 39: 221-3
6. Matsushima T, Fukui M, Kitamura K, Soejima T, Ohta M, Okano H. Mixed colloid cyst-xanthogranuloma of the third ventricle. A light and electron microscopic study. Surg Neurol. 1985. 24: 457-62
7. Miranda P, Lobato RD, Ricoy JR, Lagares A, Ramos A. Xanthogranuloma of the choroid plexus of the third ventricle: Case report and literature review. Neurocirugia (Astur). 2005. 16: 518-22
8. Swaminathan G, Jonathan GE, Patel B, Prabhu K. Xanthogranulomatous colloid cyst of the third ventricle: Alter your surgical strategy. Neuroradiol J. 2018. 31: 47-9
9. Tamura Y, Uesugi T, Tucker A, Ukita T, Tsuji M, Miyake H. Hemorrhagic colloid cyst with intraventricular extension. J Neurosurg. 2013. 118: 498-501
10. Webb AJ, Gillies MJ, Cadoux-Hudson TA. Acute vasospasm following transcallosal resection of a xanthogranulomatous colloid cyst of the 3rd ventricle. Clin Neurol Neurosurg. 2010. 112: 512-5
11. Wiot JG, Lukin RR, Tomsick TA. Xanthogranuloma of the third ventricle. AJNR Am J Neuroradiol. 1989. 10: S57-