- Chief of Neurosurgical Spine/Education, NYU Winthrop Hospital, Mineola, New York, USA
Correspondence Address:
Nancy E. Epstein
Chief of Neurosurgical Spine/Education, NYU Winthrop Hospital, Mineola, New York, USA
DOI:10.4103/sni.sni_243_17
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Nancy E. Epstein. Nursing review of cervical laminectomy and fusion. 11-Dec-2017;8:300
How to cite this URL: Nancy E. Epstein. Nursing review of cervical laminectomy and fusion. 11-Dec-2017;8:300. Available from: http://surgicalneurologyint.com/surgicalint-articles/nursing-review-of-cervical-laminectomy-and-fusion/
Abstract
Background:Cervical radiiculopathy/nerve root compression, myelopathy/cord compression are variously attributed to stenosis/narrowing of the spinal canal [anterior/posterior (AP) to less than 10 mm is defined as absolute stenosis, and 13 mm as relative stenosis]. Additional pathology includes disc herniations, ossification of the posterior longitudinal ligament (OPLL), and ossification of the yellow ligament (OYL). Patients, typically over 60 years of age, may present with severe myeloradicular syndromes requiring multilevel laminectomies and posterior instrumented fusions.
Methods:Patients typically first undergo magnetic resonance imaging (MRI) studies of the cervical spine that best demonstrate soft tissue pathology; spinal cord and/or nerve root compression in three dimensions (AP/coronal (front/back), lateral (side), and axial (cross section)). Computed tomography (CT) studies better define ossification/calcific changes contributing to stenosis, including OPLL and/or OYL.
Results:If there is multilevel cervical pathology and an adequately preserved cervical lordosis (curvature with the neck), a cervical laminectomy may provide adequate cord/root decompression. Performed under intraoperative monitoring, the laminae (bones cover the back of the cervical spine), spinous processes (midline bony protuberant structures), and OYL may be directly removed. Posterior fusions, utilizing varying instrumentation/constructs may prevent reversal of the lordosis (kyphosis: curve angled forward) and re-tethering of the spinal cord.
Conclusions:Patients with myeloradiculopathy (cord/root compression) and multilevel cervical stenosis attributed to disc herniations, OPLL, and/or OYL with an adequate lordosis may require multilevel laminectomy and an instrumented fusion.
Keywords: CT images, MR studies, multilevel cervical laminectomy, myelopathy, posterior cervical fusions, radiculopathy
INTRODUCTION
Patients who present with cervical radiculopathy/root compression, and myelopathy/cord compression attributed to stenosis and disc herniations, ossification of the posterior longitudinal ligament (OPLL), and ossification of the yellow ligament (OYL) may require multilevel laminectomies and posterior instrumented fusions [Figures
Figure 1
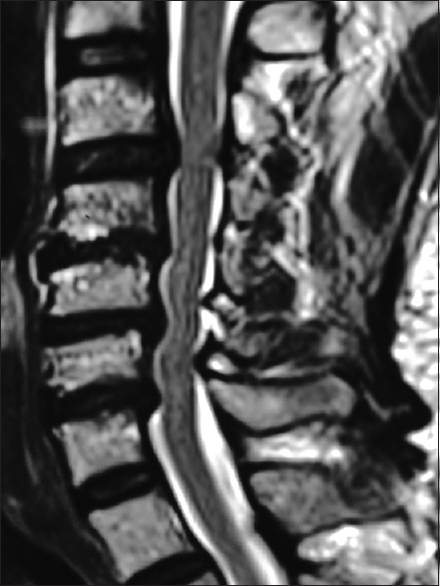
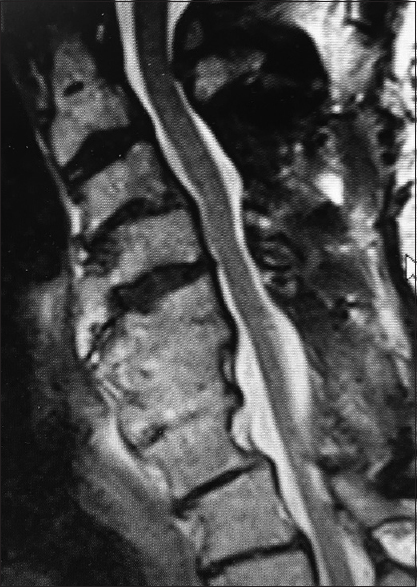
The preoperative T2-weighted sagittal MR best documents soft tissue pathology, including in this study the multiple sites of cord compression. Note the ventral thecal sac and compression from hypertrophy/ossification of the posterior longitudinal ligament (OPLL) anteriorly at C3–C4, C4–C5, C5–C6, and C6–C7. There is also dorsolateral intrusion due to laminae shingling and ossification of the yellow ligament at the C3–C4, C5–C6, and C6–C7 levels
Figure 4
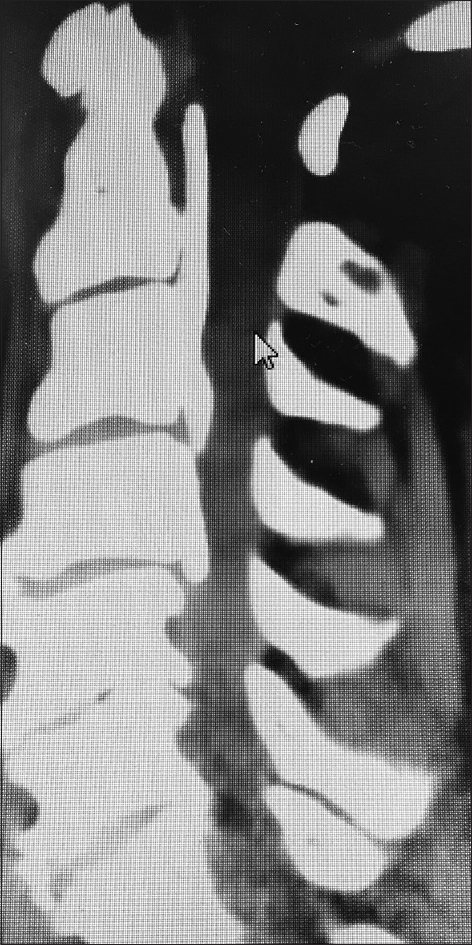
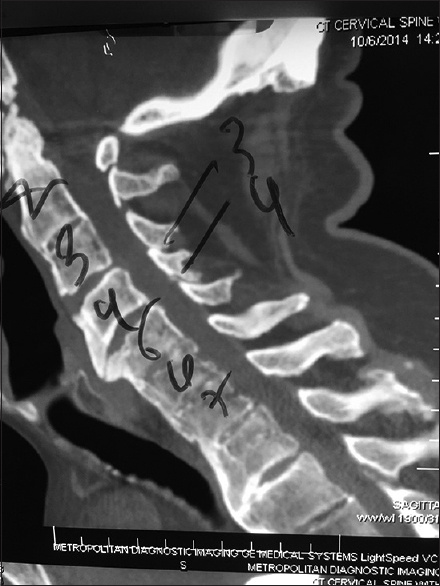
This midline sagittal bone window 2D-CT demonstrated spontaneous fusion at the C2–C3 level and from C4 downward, consistent with ossification of the anterior longitudinal ligament and/or DISH (diffuse idiopathic skeletal hyperostosis). Note on the MR and CT there was maximal stenosis at the C3–C4 level, the only level that was not fused. Here, a laminectomy of C3 and C4 with posterior C2–C5/C6 fusion provided decompression/stabilization
Figure 5
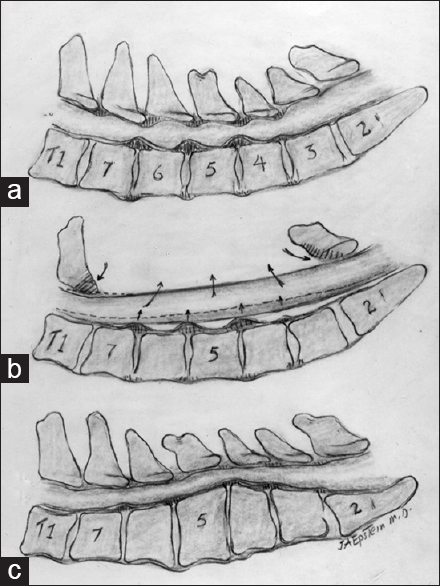
This illustration (Joseph A. Epstein, M.D. Copyright Nancy E. Epstein M.D) documented (a). multilevel cervical stenosis with ventral disc/osteophyte and dorsolateral OYL and laminar shingling contributing to diffuse C4/C5, C5/C6, and C6/C7 cervical stenosis. (b) A laminectomy C3–C7 alone was performed and provided adequate decompression without the need for fusion. (c) A kyphotic cervical spine; the reversed lordosis would not be appropriate for a laminectomy
Figure 6
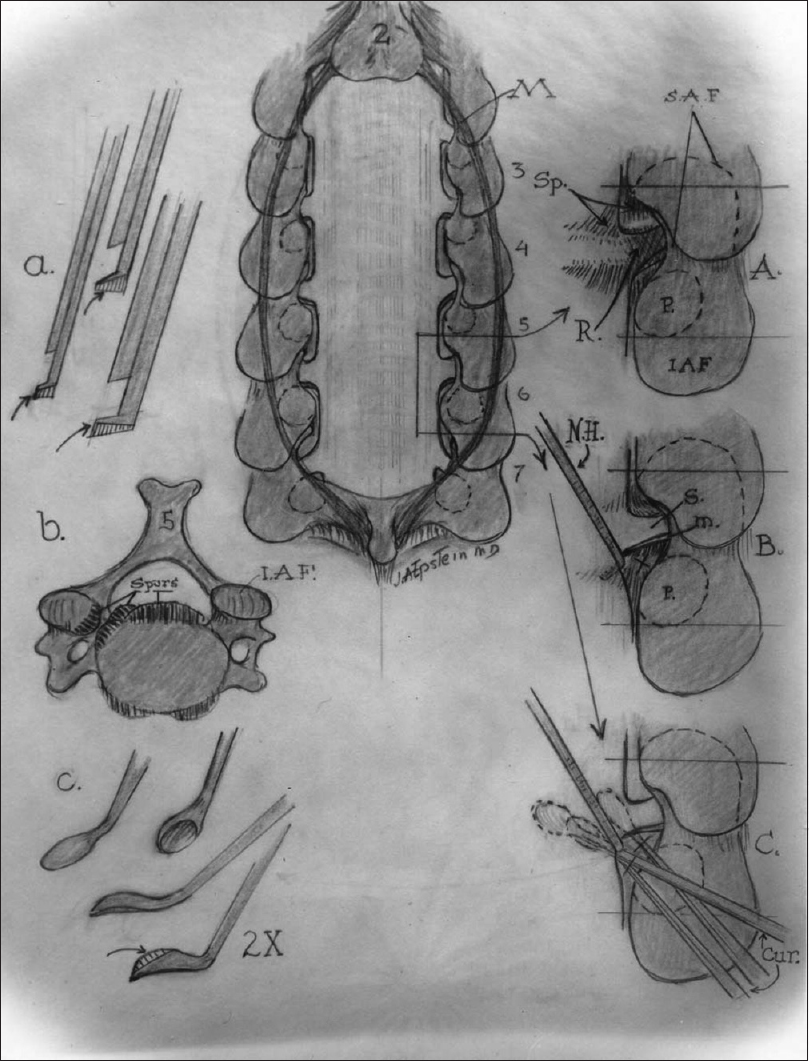
The images on the left show (a) the filed-down Kerrison punches used for bone removal, the (b) ventral osteophytes in the cervical spinal canal, and (c) the down-biting curettes used to remove these osteophytes in the cervical spine. The midline image is of a cervical laminectomy C3–C7 with (A) medial facetectomy foraminotomy, (B) foraminal root elevation with a nerve hook, and (C) use of the down-biting curette to remove foraminal osteophytes
Figure 7
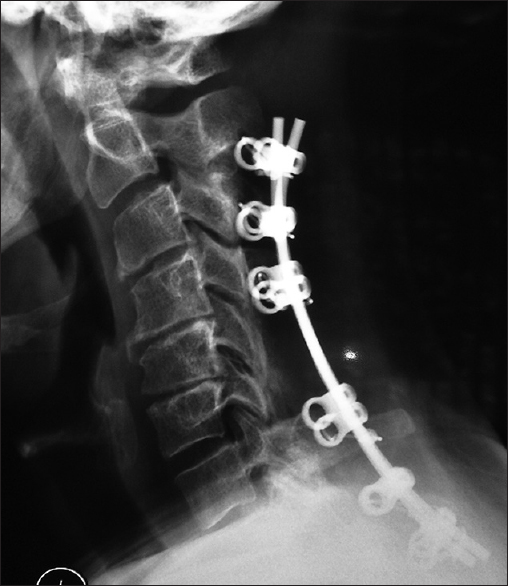
This lateral X-ray shows the vertex/rod/eyelet/titanium cable system affixed to the spinous processes superiorly of C2, C3, C4, the laminectomy C5, C6, and the fusion of the C7, T1, and T2 spinous processes inferiorly. Note in this case due to marked osteoporosis the C2 cables have pulled out of C2; this patient was still sent on to fuse in a Miami J CTO (Cervical Thoracic Orthosis) for 4 postoperative months
MATERIALS AND METHODS
Clinical presentation with radiculopathy, myelopathy, or myeloradiculopathy
Patients with cervical spine disease may present with cervical radiculopathy, myelopathy, or both (myeloradiculopathy). Those with cervical radiculopathy often present with pain, numbness, tingling, and weakness in a specific nerve root distribution. Those with myelopathy/cord compression, may have no pain at all, but experience more diffuse numbness, tingling, and weakness involving entire limbs and or 2-4 extremiites.
Neurological examination in patients warranting cervical surgery for myeloradiculopathy
The indications for cervical spine surgery include “significant” neurological deficits that correlate with specific and “significant surgical” radiographic findings. Rarely, asymptomatic patients with severe cord compression on MR/CT studies may require prophylactic cervical surgery; for those with the most severe stenosis, approximately 10% of these patients may otherwise present with irreversible quadriplegia following even minor traumatic events. Most patients, however, should exhibit a “significant” motor deficit, accompanied by appropriate reflex and/or sensory changes. Those with varied lateral/foraminal (off to the side) discs/stenosis, involving a single nerve root, may just warrant a focal lamnoforaminotomy. This is most frequently observed at the following levels (descending order of frequency): the C6 root at the C5–C6 level, the C7 root at the C6–C7 level, and the C5 root at the C4–C5 level. For those with cord compression/myelopathy, disc disease, OPLL, and/or OYL, resulting in cord compression with/without root involvement, a laminectomy may be warranted. If there is instability, an accompanying fusion may also be indicated.
MR scans best documents cervical root and cord compression
MRI best identifies cord and/or root compression (soft tissue detail). Images are provided in three views – anterior/posterior (AP)/coronal (from the front to the back), sagittal (lateral or side view), and axial (cross-section view) [
CT best documents ossiffic/calcific changes attributed to cervical spinal stenosis, OPLL, and OYL
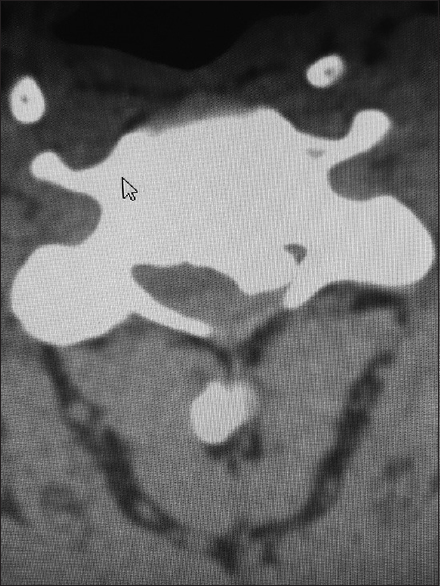
Cervical CT studies better document, with a positive image (hyperdense), the presence of ossification/calcification associated with stenosis, OPLL, and OYL [Figures
Surgery for nerve root deficits: Laminoforaminotomy
For very lateral pathology (off to the side and not compressing the cord but impinging on a nerve root), a laminoforaminotomy may be performed. This includes, on one level, removal of a portion of the lamina above and below the disc, with excision of the medial (inside) portion of the facet joint. This is very different from anterior cervical diskectomy/fusion where anterolateral cord/root compression warrants an anterior approach requiring graft/instrumented fusion.
Surgery for cervical cord compression: Multilevel laminectomy/fusion
Typically, these older patients (e.g., over the age of 60) exhibit multilevel anterior spondylosis (not soft disc herniations but calcified/ossified spurs/discs), OPLL, with additional lateral/foraminal spurs/discs, posterolateral OYL, synovial cysts, and laminar shingling. They often have severe “painless” myelopathic deficits characterized by significant quadriparesis and “useless hands.” The latter syndrome is defined by marked loss of function in either one or both hands. Notably, some of these patients have a “double crush syndrome” characterized by severe myeloradiculopathy plus carpal tunnel syndrome (CTS). Complete radiological work up includes MR and CT studies of the cervical spine along with AP, lateral, and flexion/extension cervical X-rays. The latter dynamic films often demonstrate significant multilevel instability. For patients with multilevel cord compression/instability with an adequate cervical lordotic curvature (curve with the neck), a multilevel laminectomy may decompress the cervical spinal cord and may be followed by a fusion.
Performing multilevel cervical laminectomy: Multiple preoperative, perioperative, and postoperative concerns
Preoperative cardiovascular clearance
As these patients are typically over the age of 60, they often present with several major comorbid factors. Medical/cardiology clearance is warranted to rule out significant cardiovascular disease as these patients are often overweight with accompanying major comorbid risk factors; e.g., diabetes, hypertension, and pulmonary, and other pathologies.
Cessation of all blood thinners and potential consequences
For patients about to undergo cervical laminectomies/fusions, cessation of all antiplatelet aggregant therapy and/or anticoagulants is critical. For example, NSAIDS (nonsteroidal anti-inflammatories) and antiplatelet aggregants (including Aspirin) should be stopped 3 weeks ahead of time. Note that our medical/hematological colleagues tell us the platelets are regenerated in 10 days; however, our eperience is that in fact, it requires a longer period of time for adequate platelet regeneration to occur. Coumadin should be stopped 5–7 days preoperatively, whereas Xarelto, Eliquis and other newer anticoagulants may require only a day or two. Foods with Vitamin E (e.g., nuts including almond milk), and other blood-thinning vitamins/supplements [Omega-3's (fish/fish oils), Co-Q 10, almond mild/nuts with Vitamin E] must be stopped optimally 3 weeks ahead of time. Note, patients and their medical physicians should also be counseled that these medications/vitamins etc., cannot be restarted until about 4–6 weeks postoperatively, as otherwise, there is an increased risk of postoperative wound breakdown, hematoma, or seroma. Some patients with cardiac/carotid/peripheral vascular/other stents and/or other pathology might simply not be surgical candidates.
Risk of postoperative visual loss with cervical laminectomy (in the prone position)
Patients with significant ophthalmological disease and all other patients should be warned, that with surgery performed in the prone position, one of the major risks is postoperative visual loss (POVL). This is most commonly attributed to nonarteritic ischemic optic neuropathy (ION), but other factors contributing to POVL may include; intraoperative hypotension, direct compression, acute increased intraocular pressure (e.g., with acute closure of the angle in glaucoma), and ischemia (thrombosis). To avoid POVL while performing cervical surgery in the prone position, we use an arterial line to avoid hypotension, elevate the head of bed (HOD) 10 degrees (to decrease eye pressure), and a use a three-pin head holder to avoid any direct compression on the eyes or the face.
Prophylactic measures to prevent posterior cervical infections
The incidence of posterior cervical surgical infections ranges from 3–5% in different studies, and is much higher than the <0.5% typically cited for anterior cervical surgery. To avoid posterior cervical infections, patients are asked to start Hibiclens washes to the neck front/back, and below (just avoiding the groin due to burning from the alcohol) all the way to the feet, 2 weeks prior to surgery. They must, however, avoid the eyes and ears. Additionally, they recieve from preoperative testing, Hibiclens sponges for washing the night before and the morning of surgery. Moreover, preoperative antibiotics, repeated intraoperative antibiotics every 4 hours, intraoperative antibiotic irrigation every 15 minutes (Bacitracin and Polymyxin B Sulfate), and postoperative antibiotics are employed (e.g., Cefazolin in those without Penicillin allergies; with an allergy, use typically of Vancomycin). All patients also receive Silverlon dressings to the posterior cervical incision site for 1 month postoperatively while the hip graft site is covered with a clean dry dressing (e.g., steri strips rather than staples are used for closure here).
Cervical laminectomy
Intraoperative monitoring: SEP, MEP, EMG
Cervical laminectomies are performed utilizing intraoperative spinal cord and nerve root monitoring. As the cervical spinal cord may be compromised anteriorly, posteriorly, laterally/foraminally, or circumferentially, intraoperative monitoring must provide 360 degree coverage. Prior to induction and surgery and prior to turning the patient prone, we institute intraoperative monitoring. This warrants obtaining baseline somatosensory (SEP), motor evoked (MEP), and electromyograhic (EMG) potentials to help avoid incurring new intraoperative neurological deficits. SEPs monitor the back of the spinal cord; specifically, they track the function of the posterior columns (e.g., subserving position/vibration appreciation). When changes are noted either in the latency (how long it takes the wave to be completed) and/or amplitude, (the height/strength) of the electrical impulses/waves, certain intraoperative resuscitative measures may be warranted. As soon as the patient comes into the operating room, under mild sedation and before intubation, baseline SEP in the upper and lower extremities (upper; medial/ulnar; lower; posterior tibial) are obtained with skin surface electrodes. MEP that monitor the motor cells/corticospinal tracts of the anterior cord are also obtained using a transient bolus of Propofol for placement of the three-pin head holder and Foley catheter. EMG recordings evaluate the status of the peripheral nerves/muscular innervation, and are monitored by placing electrodes in specific muscle groups in both the upper and lower extremities. As MEPs and EMGs are performed throughout the duration of surgery, muscular paralysis (paralytics will make them drop out) must be avoided. Anesthesia typically uses total intravenous anesthesia (TIVA) without motor blockade.
Awake intubation and awake prone positioning
Patients with severe cervical stenosis typically warrant awake nasotracheal intubation and positioning. Using a fiberoptic device, nasal or endotracheal fiberoptic intubation may be completed applying a local anesthetic. Alternatively, in some cases, the glidescope may be used (e.g., for posterior surgery alone). As flexion/extension must be avoided during intubation, a collar is often placed and later used when the patient is turned prone. The three-pin head holder is then affixed to the Mayfield system. Throughout the case, MEP, SEP, and EMG baselines are matched to any new intraoperative findings. If significant changes occur, different resuscitative measures may be needed.
Technical performance of cervical laminectomy
Following induction in the prone position, laminectomy is initiated by first preparing and draping the posterior cervical region. The posterior cervical incision is marked from the inion (bump on the back of the head) to the T2 level (lower-most spinous process on the neck/between the shoulders). The incision is made (after appropriate antibiotics were given), exposing the cervical spinous processes of C2-T2. A clamp is placed on C2 for X-ray confirmation/localization. Next, a retractor is placed bilaterally with large cottonoids protecting the skin margins, and the laminae/spinous processes are skeletonized of all soft tissues. The microscope is brought into the field, and the laminectomy levels and respective laminae are initially scored with an AM 33-diamond burr. Next, the laminae on the left side are thinned to 1 mm just medial (inside) to the facet joints. For example, a laminectomy of C5, C6, and C7 would warrant shaving the laminae of C5, C6, C7 and the facet joints of C4/C5, C5/C6, C6/C7, and C7/T1 [Figures
Fusion technique alternatives
Vertex/rod/eyelet fusion
Following laminectomy, one fusion technique involves the application of vertex/rod/eyelet/titanium cable system applied to remiaing intact spinous processes [
Lateral mass screws and laminoplasty
There are multiple alternatives for posterior cervical fusion. Some use lateral mass screws that were just recently approved by the FDA. However, the best CT cadaveric studies using Stealth-CT guidance previously documented that 10% of these screws were critically located (e.g., through nerve roots or the vertebral artery) and another 15% were semi-critically located (nearly traversing major roots or arteries). Other constructs include different laminoplasty techniques developed to keep the decompressed side opened (e.g., note the other side is thinned and bent backward). Laminoplasty constructs have used various plates, spacers etc., to maintain an open door and overall, cervical decompression.
Fusion mass; “gold standard” iliac crest autograft
The “gold standard” for the posterior cervical fusion remains iliac crest autograft. This requires that iliac crest autograft be harvested through a separate incision, and many studies have exaggerated the morbidity associated with this harvest. Nevertheless, iliac autograft and autogenous bone marrow aspirate (BMA) may be readily obtained and combined with the lamina autograft harvested during the laminectomy. The fusion mass may then be applied posterolaterally. The bone may be morcellized (avoid the bone mill/they makes a paste that irrigates away from the site) and supplemented/supplanted with bone graft extenders as needed.
Bone graft extenders: Supplements or substitutes
Bone morphogenetic protein (Infuse, Medtronic, Memphis, USA); bone graft substitute not FDA approved for use in the posterior cervical spine
In 2002, BMP/Infuse was released and approved by the FDA only for use with the LT Cage (LT: Lumbar Tapered Cage Device, Medtronic, Memphis, USA) to perform anterior lumbar interbody fusions (ALIF).[
Vitoss (Stryker, Kalamazoo, MI, USA) and Vitoss Vs. Nanoss (Alachua, Fl, USA) as bone graft expanders for posterior cervical fusions
Vitoss, beta tricalcium phosphate, and later Vitoss vs. Nanoss were documented to be viable bone graft expanders for use in the posterior cervical spine.[
Bone graft extender Nanoss FDA approved for posterolateral spine fusion
The bone graft expander, Nanoss, (RTI: Regeneration Technologies, Alachua, FL, USA) is presently used and FDA approved as a bone graft expander when used with autograft and autogenous BMA in the posterolateral cervical spine.[
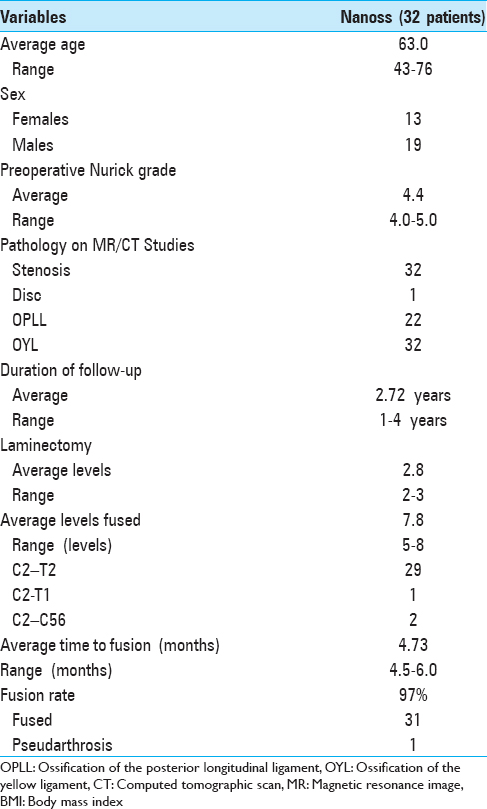
Recent experience with 32 patients undergoing multilevel laminectomy with instrumented fusions using lamina/iliac autograft and Nanoss/BMA
Cervical laminectomies (average 2.8 levels)/multilevel fusions (average 7.8 levels; vertex/rod/eyelet/titanium cable) were successfully performed in 32 patients monitored over 2.7 years; surgery required an average of 4.6 hours [
CONCLUSION
Patients with severe, multilevel cervical myeloradiculopathy, and marked cord/root compression on MR/CT studies may require multilevel cervical laminectomy/posterior fusions. Here, we explored the clinical presentation and radiographic diagnosis of cord/root compression on MR/CT studies, and reviewed how to perform a laminectomy and several types of posterior cervical fusions.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Epstein NE. A preliminary study of the efficacy of Beta Tricalcium Phosphate as a bone expander for instrumented posterolateral lumbar fusions. J Spinal Disord Tech. 2006. 19: 424-9
2. Epstein NE. Efficacy of different bone volume expanders for augmenting lumbar fusions. Surg Neurol. 2008. 69: 16-9
3. Epstein NE. An argument for traditional posterior cervical fusion techniques: Evidence from 35 cases. Surg Neurol. 2008. 70: 45-51
4. Epstein NE, Schwall GS. Costs and frequency of “off-label” use of INFUSE for spinal fusions at one institution in 2010. Surg Neurol Int. 2011. 2: 115-
5. Epstein NE. Efficacy of posterior cervical fusions utilizing an artificial bone graft expander, beta tricalcium phosphate. Surg Neurol Int. 2011. 2: 15-
6. Epstein NE. Pros, cons, and costs of INFUSE in spinal surgery. Surg Neurol Int. 2011. 2: 10-
7. Epstein NE. Complications due to the use of BMP/INFUSE in spine surgery: The evidence continues to mount. Surg Neurol Int. 2013. 4: S343-52
8. Epstein NE. Basic science and spine literature document bone morphogenetic protein increases cancer risk. Surg Neurol Int. 2014. 5: S552-60
9. Epstein NE. Commentary: Bone morphogenetic protein's contribution to pulmonary artery hypertension: Should this raise concern for patients undergoing spinal fusions with bone morphogenetic protein?. Surg Neurol Int. 2014. 5: S570-3
10. Epstein NE. Preliminary documentation of the comparable efficacy of vitoss versus NanOss bioactive as bone graft expanders for posterior cervical fusion. Surg Neurol Int. 2015. 6: S164-71
11. Epstein NE. High Posterior Cervical Fusion Rates With Iliac Autograft and Nanoss/Bone Marrow Aspirate. Surg Neurol Int. 2017. p.